 Contingency Fees Act, 1997
Contingency Fees Act, 1997
R 385.00
Pharmacy Act, 1974 (Act No. 53 of 1974)Board NoticesRules Relating to Good Pharmacy PracticeChapter 2 : Professional Standard for Services2.3 Minimum Standards for Procurement, Storage and Distribution |
| 2.3.1 | Responsibility for procurement |
The pharmaceutical aspects of the purchase of all medicinal products and related materials must be the responsibility of a pharmacist. Written policies for the procurement, storage and distribution of medicine and scheduled substances must be available in the pharmacy. Written policies must assist in ensuring:
| (a) | product availability when required; |
| (b) | that the procurement and distribution process is fully documented; |
| (c) | effective batch recall of medicines when necessary; |
| (d) | that optimal storage conditions are monitored (including during transport); |
| (e) | the safety of medicines; |
| (f) | that patients receive stock that has been suitably stored and has an expiry date that allows sufficient time for usage by the patient before the expiry date. |
| 2.3.2 | Sources of supply |
| (a) | The responsible pharmacist has a professional responsibility to exercise control over all medicinal and related products, which are purchased or supplied. |
| (b) | A purchasing policy must be in place that ensures the safety of medicine. |
| (c) | A pharmacist must not purchase, sell or supply any medicinal product where the pharmacist has any reason to doubt its safety, quality or efficacy. |
| (d) | The pharmacist must know and select suppliers by applying various quality parameters, in accordance with the MCC's standards of Good Manufacturing Practice. |
| (e) | A pharmacist must be satisfied that both the supplier and the source of any medicine purchased are reputable and recorded with Council. Due regard must be paid to the storage conditions before purchase and to the labels, leaflets, appearance, origin and subsequent chain of supply of the medicine concerned. |
| (f) | A MCC registration number must appear on the original container of every medicine in the facility, with the exception of unregistered medicines for which authority for the use thereof has been granted by the MCC. |
| (g) | Stock rotation must always be done on the 'FIRST EXPIRY, FIRST OUT' (FEFO) or 'FIRST IN — FIRST OUT' (FIFO) basis. Stock that expires first or is received first (where expiry dates are not available) must therefore be used first. |
| (h) | Patients must receive stock that has been stored suitably and has an appropriate expiry date. |
| 2.3.3 | Safe systems of work |
A pharmacist must take all reasonable steps to ensure that working conditions are so arranged as to protect the safety of the public and people working in the pharmacy. In adhering to this principle the following must be taken into consideration:
| (a) | Safe systems of work must be established and maintained by a pharmacist to eliminate, as far as possible, errors in any component of the pharmaceutical service. |
| (b) | Secure storage for medicines must be provided in all premises and approved store-keeping procedures and adequate stock control systems must be maintained. |
| (c) | A policy for the storage and administration of medicines in hospitals must be defined and updated regularly. A pharmacist must establish systems, and be responsible, for the professional element of the procurement of all medicinal products purchased through the hospital pharmaceutical service. |
| (d) | Within a multidisciplinary system a pharmacist is responsible for providing advice as needed on the pharmaceutical element of the procurement of surgical dressings. |
2.3.4 Medical gases
The procurement, storage and distribution of medical gases must comply with the requirements as described in the Guidance Document: Good Manufacturing Practice for Medicine in South Africa (GN R7659 of 2 May 2003), published by the MCC, which could include but not be limited to the following:
| (a) | All equipment supplied for use must be fit for its purpose and must be maintained in a manner that will ensure the safe and proper use thereof. |
| (b) | Cylinders and other containers of medical gases must be stored in accordance with the current guidelines issued by the manufacturers of the gas. |
| (c) | The supply of medical gases to a patient must be in association with treatment of a medical condition. |
| (d) | Each patient, and appropriate members of the patient's family or caregiver, must receive full and proper instruction from a pharmacist or suitably trained person in the safe care and handling of the cylinders and associated equipment. |
| (e) | To facilitate recalls of faulty oxygen giving sets, the name, type, serial number and location of each regulator must be recorded and held in the pharmacy. |
| (f) | The responsible pharmacist in an institutional pharmacy must ensure that an appropriate Standard Operating Procedure is in place for the handling of medical gases in the institution. |
| 2.3.5 | Minimum standards for the procurement, storage and distribution of thermolabile pharmaceutical products |
| 2.3.5.1 | Introduction |
Thermolabile pharmaceutical products are defined as all products which require constant cold storage at product specific temperatures below room temperature. This also includes vaccines which are normally stored between 2ºC and not exceeding 8ºC. 'Cold chain products' bears a corresponding meaning.
Storage, supply and distribution forms part of the supply chain management of thermolabile pharmaceutical products. All pharmacists are responsible for the effective, efficient and safe handling, storage and distribution of such products. These standards set out appropriate steps for meeting this responsibility.
Handling and storage of thermolabile pharmaceutical products must be in accordance with procedures, which must be established and designed to prevent contamination, deterioration of the goods, and damage to packs and/or confusion of products. Particular care must be given to maintaining the integrity of ingredients and seals on packs. Attention must be paid to instructions from the manufacturer relating to handling or storage of the goods. Distribution systems chosen to deliver thermolabile pharmaceutical products from the manufacturer/importer to the end user must take into account basic operational parameters, including timeliness and accountability.
Importers must take all reasonable measures to ensure that thermolabile pharmaceutical products are not mishandled or exposed to adverse storage conditions at ports of entry.
Storage, supply and distribution of thermolabile pharmaceutical products must be in accordance with the provisions of the Medicines and Related Substances Control Act, 101 of 1965 and the manufacturer's specification.
| 2.3.5.2 | Procurement |
Procurement of thermolabile pharmaceutical products must be performed in terms of the Minimum standards for procurement, storage and distribution as detailed in Rule 2.3 of the Rules pertaining to good pharmacy practice.
| 2.3.5.3 | Storage area |
Storage areas may include inter alia cold rooms, refrigerators and freezers. Thermolabile pharmaceutical products require controlled temperature storage and therefore must be identified on receipt and be stored in accordance with written instructions. Temperatures must be monitored and recorded twice daily. Records must be reviewed regularly. Controlled temperature storage areas must be equipped with temperature recorders. Control must be adequate to maintain all parts of the area within the specified temperature range. This control is essential in maintaining the quality of thermolabile pharmaceutical products and in helping to protect the end user from substandard or ineffective thermolabile pharmaceutical products as a result of inadequate control.
| (a) | Thermolabile pharmaceutical products must be stored in a storage area, refrigerator or cold room, in a temperature regulated environment as per the information on the manufacturer's product label indicating which temperature must be maintained at all times. |
| (b) | The storage area must be large enough to allow for orderly arrangement of products, to permit air circulation especially between shelving and for proper product rotation. If it is filled to capacity, the effect on temperature distribution must be investigated. |
| (c) | The storage area must be kept clean. Internal air temperature distribution must be mapped on installation of the storage area while empty and thereafter fully stocked. This must be done annually under conditions of normal use. Thermolabile pharmaceutical products must not be stored in areas shown by temperature mapping to present a risk (e.g. in the airflow from the refrigeration unit). |
| (d) | All storage areas, such as refrigerators or cold rooms must be properly maintained in order to maintain the factory standards for such storage areas. Proof of maintenance must be provided. |
| (e) | Condensation from chillers must not be collected inside the storage area, and no condensation from chillers may collect or drip onto the products. |
| (f) | A suitable number of temperature recording instruments that complies with or meets WHO specifications, being at least a logging device, must be installed to record temperatures and to provide temperature and profiles as per the temperature mapping of the storage area. Monitors that comply with or meet WHO specifications, must be adequate to monitor and record temperature ranges in all parts of the area within the specified temperature range. |
| (g) | Temperatures must be monitored and recorded at least twice daily, with a minimum of seven-hour interval and the records from such monitoring must be reviewed daily. |
| (h) | Large commercial refrigerators and walk-in cold rooms must be monitored with an electronic temperature-recording device that measures load temperature in one or more location, depending on the size of the unit. |
| (i) | In the monitoring of large commercial refrigerators and walk-in cold rooms, portable data-loggers that can be downloaded onto a computer may be used instead of a fixed device. |
| (j) | The refrigerator, cold room or freezer must be connected to a standby generator or other emergency power system to ensure uninterrupted power supply in the event of power failure. |
| (k) | The refrigerator, cold room or freezer must be connected to an alarm system and/or warning system in the event of a power failure or if the storage area temperature limits are exceeded. |
| (l) | Any recording devices/instruments must be calibrated annually against a certificated standard. |
| (m) | The refrigerator, cold room or freezer must be clearly designated and appropriately signed to store exclusively thermolabile pharmaceutical products. |
| (n) | Within a community or institutional pharmacy the storage area must be inside the pharmacy and must be easily accessible to the pharmacist and pharmacy support personnel and other authorised healthcare professionals. |
| (o) | Within a health facility (other than a pharmacy), any storage area for thermolabile pharmaceutical products must be easily accessible to the authorised healthcare professionals. |
| 2.3.5.4 | Distribution |
A distribution system must have in place:
| (a) | a comprehensive quality system; |
| (b) | a process for continual quality improvement; |
| (c) | an ambient and cold chain distribution strategy; |
| (d) | a risk assessment programme. |
Thermolabile pharmaceutical products must be transported by appropriately specialised means in such a way that they are secure and are not subjected to unacceptable degrees of heat/cold.
| (a) | Packaging system of thermolabile pharmaceutical products, for purposes of distribution must be quality assured to ensure that it occurs within the cold room environment, fulfils the manufacturers' specifications requirements, is thermally designed and validated, and is related to Temperature Profile(s)/Logistic history. |
| (b) | There must be clear, visible labelling on the packaging with instructions regarding storage conditions, special precautions and warnings for the shipment. |
| 2.3.5.5 | Transportation |
| (a) | Transportation of thermolabile pharmaceutical products must be in such a way that it is secure and the temperature is maintained to product specifications. |
| (b) | Mode(s) of transportation must be approved for transporting thermolabile pharmaceutical products. Examples include refrigerator trucks, cars, ships, and containers. Thermolabile pharmaceutical products shall be transported in any mode(s) of transportation which is permanently enclosed and sealed. No open vehicles shall be permitted for purposes of transporting thermolabile pharmaceutical products. |
| (c) | In the event of the mode(s) of transport not being specific for the transportation of thermolabile pharmaceutical products, the specialised packaging like validated cooler bag packaging must be used. |
| (d) | For purposes of transportation, the route must be planned and assessed and/or validated to ensure that delays and/or exposure to extreme temperatures are correctly assessed. Transportation between South Africa and other neighbouring countries and within South Africa, due to large geographical areas, must be treated as unique in terms of the range of temperatures that the thermolabile pharmaceutical products may experience. |
| (e) | The transport must be clean and free from all forms of contamination, inter alia rats, vermin, birds, fungi, and mites. |
| (f) | During transportation thermolabile pharmaceutical products must not be packaged with non-pharmaceutical items or containerised with any other goods (for example food and beverages which may also require refrigeration transportation), which could result in contamination. |
| (g) | The transport must have a sufficient capacity to allow for orderly storage of thermolabile pharmaceutical products during transportation. |
| (h) | Temperature data loggers, refrigeration tags, freezer tags, log tags or cold chain monitoring cards that comply with or meet WHO specifications must monitor the temperature of the loaded area of the transportation throughout the trip, and the validated cooler box packaging must have at least a temperature monitoring device that complies or meets with WHO specifications. |
| (i) | Personnel transporting thermolabile pharmaceutical products must be appropriately trained for cold chain management and shall provide the suitable documentation as proof for this function and they must ensure that the correct procedures are followed to maintain the cold chain within the manufacturer's specification. |
[Rule 2.3.5.5(i) substituted by Board Notice 193, GG 40522, dated 23 December 2016]
| (j) | At any stage of transportation, a delivery document must show evidence that the transport requirements, inter alia temperature control, have been met. |
| (k) | Damage to containers or any other event or problem which occurs during transit must be reported to and recorded by the Responsible Pharmacist of the distributing pharmacy. Upon arrival the person responsible for the transportation of the thermolabile pharmaceutical products must inform receiving personnel, pharmacists, or other authorised healthcare professionals, that the package includes thermolabile pharmaceutical products and that they require immediate attention. |
| 2.3.5.6 | Receiving |
Thermolabile pharmaceutical products must be identified on receipt and be stored in accordance with written instructions for purposes of stock management within the shortest possible time from offloading.
| (a) | The receiving area must protect deliveries from bad weather during the unloading of thermolabile pharmaceutical products. |
| (b) | The receiving area must be separated from the storage area. |
| (c) | Upon arrival of thermolabile pharmaceutical products, the receiving personnel must do spot checks and inspect the delivery vehicle to ensure product integrity with regards to the following: |
| (i) | product security, |
| (ii) | that the product has not been tampered with and that there are no damaged containers, |
| (iii) | that products were protected from weather, |
| (iv) | and that there is no risk for contamination of products. |
| (d) | The delivery document must be reviewed for evidence that transportation requirements, inter alia temperature control, have been met. |
| (e) | Check temperature data loggers, refrigeration tags, freezer tags, log tags or cold chain monitoring cards to ensure the temperature history of the transport and the temperature history of the thermolabile pharmaceutical product being transported were maintained within in limits. |
| (f) | If any discrepancies are identified, they must all be documented. In addition, the supplier must be notified immediately and the thermolabile pharmaceutical products must be identified and segregated. |
| (g) | A Standard Operating Procedure for receiving of thermolabile pharmaceutical products must be used to ensure these products are within manufacturer specific temperature range during the receiving process. |
| (h) | Quality assessment sampling requiring laboratory testing is required for the received thermolabile pharmaceutical products within a manufacturing pharmacy before they are taken to the main store facility. |
| (i) | Quality assessment sampling requiring observation for damaged products is required for the received thermolabile pharmaceutical products within a wholesale, community or institutional pharmacy before they are taken to the main store facility. |
| (j) | Delivery documents must be signed off on temperature data and condition of other control devices used. |
| (k) | The thermolabile pharmaceutical products must be removed from the transportation container or cooler bag prior to storage in the main store area to prevent temperature deviation. |
| 2.3.5.7 | Documentation |
| (a) | Documentation is critical. Each step of the supply chain must follow established protocols in order to maintain proper records. |
| (b) | Customs delays may occur due to inaccurate or incomplete customs documentation, therefore guidelines for creating a commercial invoice must be followed to ensure the proper verbiage, number of copies, and other details. |
| (c) | Each time the process does not conform to the procedure, the event must be properly documented, investigated and corrected so that the deviations do not occur on future transportation. |
| 2.3.5.8 | Personnel |
| (a) | All persons involved in the procurement, storage and distribution of thermolabile pharmaceutical products must have the education, training, experience or combination of these elements that will allow them to effectively discharge this responsibility and be capable of meeting these requirements. This training must be documented. |
| (b) | Procedures and conditions of work for employees and other persons having access to thermolabile pharmaceutical products must be designed and managed to minimise the possibility of such pharmaceutical products being in possession of unauthorised persons. |
| (c) | All persons involved in the procurement, storage and distribution of thermolabile pharmaceutical products must have the ability/skill appropriate to their responsibility, for ensuring that thermolabile pharmaceutical products are handled, stored and distributed in accordance with the required minimum standards. |
| (d) | There must be an adequate number of competent persons involved in all stages of the distribution of thermolabile pharmaceutical products in order to ensure that the quality of these products is maintained. |
| (e) | Persons involved in the procurement, storage and distribution of thermolabile pharmaceutical products must be supplied with appropriate personal protective equipment and with specialised protective garments suitable for the activities that they perform. |
| (f) | Material safety data sheets must be accessible to any staff member that requires the information. A Spillage Handling Standard Operating Procedure must be available, in accordance with National Environment Management Act (1978) and other related prescripts. |
| (g) | Appropriate procedures relating to personal hygiene and sanitation, relevant to the activities to be carried out, must be established and observed. Such procedures must cover, inter alia, health, hygiene and clothing of personnel. |
| 2.3.5.9 | Disruption in the procurement, storage and distribution of thermolabile pharmaceutical products (cold chain) |
When there is a disruption in the storage and or distribution of thermolabile pharmaceutical products or a disruption is reasonably suspected:
| (a) | In the event that there is disruption in the cold chain, the designated responsible person must be informed and appropriate steps taken to manage the situation. |
| (b) | Actions must comply with manufacturer's documented advice, where possible and where available. |
| (c) | An incident report and root cause analysis investigation must be completed to ensure lessons are learned to prevent reoccurrence. |
| (d) | Pharmacies and authorised healthcare professionals must have business continuity plans for storing thermolabile pharmaceutical products in the event of refrigerator breakdown, loss of electricity supply, defrosting or other disruptions to the cold chain, which must be implemented immediately to prevent loss. |
| (e) | Refrigerator temperature must be recorded by noting the current reading and recording the maximum and minimum temperatures. |
| (f) | If the temperature of the storage area has deviated from the product specific temperature requirement, stock must be moved to an alternative cold storage area. |
| (g) | In the event of moving stock, the stock so moved must be segregated by packing separately and marked, indicating 'Batch Number(s) involved in a potential incident' — 'do not use until authorised' and dated to make sure the implicated stock can be identified and kept separate. |
| (h) | Where immediate removal is not possible, the storage area must be kept closed to maximise temperature control. |
| (i) | Monitoring of the temperature must be maintained on at least an hourly basis and recorded up until the point of restoration to working order of the storage area or removal and transfer to another cold store. |
| (j) | Check for evidence of exposure of the thermolabile pharmaceutical products for deviations in temperature and establish how long the products have been stored outside of the specified temperature requirements. |
| (k) | Refer to the manufacturer for advice on stability as a result of temperature deviation and report the occurrence on an incident report form. |
| (l) | Where necessary record and quarantine the thermolabile pharmaceutical product for destruction in line with the minimum standards for destruction and disposal of medicines and scheduled substances. |
| (m) | If advised that the products are safe for use then mark as 'Use first' and date, such products must then be used before any other stock of the same product. |
[Rule 2.3.5 substituted by Board Notice 50, GG 38511, dated 27 February 2015]
| 2.3.6 | Maintenance of the refrigerator |
| (a) | The temperature of the refrigerator must be maintained between 2°C and 8°C. |
| (b) | The temperature of the refrigerator must be monitored and charted twice daily. |
| (c) | A WHO approved dial thermometer or alcohol or mercury thermometer must be used. (NOTE: A minimum/maximum thermometer must not be used.) The thermometer must be hung from the middle shelf of the refrigerator. |
Figure 6: A WHO approved dial thermometer or alcohol or mercury thermometer
| (d) | If the power is off for any length of time, the refrigerator should not be opened until the power supply is restored. |
| (e) | The refrigerator must be cleaned and defrosted at least once a month. |
| (f) | The cold chain must be maintained when the refrigerator is cleaned. Cooler boxes should be used to maintain the cold chain. |
| (g) | The following procedures must be followed when the refrigerator is cleaned: |
| (i) | The inside of the refrigerator must be cleaned with an appropriate cleaning solution and wiped dry. |
| (ii) | The door gasket must be cleaned, especially along the bottom edge on upright units. |
| (iii) | When applicable it should be determined whether the freezing compartment needs defrosting (more than 10 mm of ice on the evaporator) and be defrosted if necessary. |
| (iv) | The condenser coil on the back of the refrigerator should be cleaned and dust removed from the compressor. |
| (v) | It must be ensured that the door closes tightly. |
2.3.7 Storage of vaccines
| (a) | All vaccines and diluents must be stored in the refrigerator between 2°C and 8°C in a pharmacy that issues to the end-user or clinics. |
| (b) | During distribution all vaccines must be transported in insulated containers at a temperature between 2°C and 8°C. |
| (c) | Various aids can be used to monitor the temperature of vials, e.g. cold chain monitors cards (CCMs) and vaccine vial monitors (VVMs). |
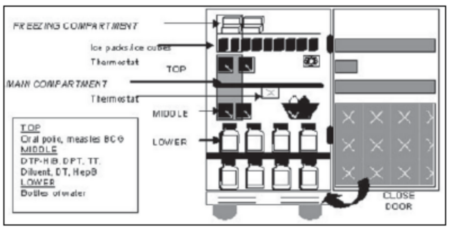
| (d) | Correct packing of vaccines and diluents in the refrigerator is vital if they are to be kept at safe temperatures. Figure 7 indicates how a refrigerator should be packed. |
Figure 7: Correctly packed refrigerator
| (e) | Vaccines must not be kept: |
| (i) | In the door compartments of domestic refrigerators; |
| (ii) | In such a way that they can come into contact with the evaporator plate, i.e. not close to the back or top of the refrigerator. |
| (f) | Vaccines must not be stored for longer than the specified storage period. |
| (g) | Vaccines must be stored in such a way that they cannot be confused with other thermolabile drugs. |
| (h) | Diluents should be at the same temperature as the vaccine at the point of use. |
| (i) | Only the designated diluents must be used for specific vaccines. |
| (j) | All vaccines must be protected by insulated packing during distribution. The containers must be filled with sufficient ice packs to give the container twice the length of cold life anticipated for a particular journey. For example: if a courier service guarantees to deliver a package within 24 hours, that package must have a cold life of a minimum of 48 hours. All ice packs must be properly conditioned (the ice must rattle in the water inside the ice pack to prevent accidental freezing of the vaccines). |
| (k) | Shake Test: |
The Shake Test is designed to determine whether adsorbed vaccines (DPT, DT, Td, TT, DTP-Hib or Hepatitis B) have been frozen. After freezing, the vaccine is no longer a uniform cloudy liquid, but tends to form flakes which gradually settle to the bottom after the vial has been shaken. Sedimentation occurs faster in a vaccine vial which has been frozen than in a vaccine vial from the same manufacturer which has never been frozen.
Note that individual batches of vaccine may behave differently from one another. Therefore, the test procedure described below should be repeated with all suspect batches.
Test procedure:
| (1) | Prepare a frozen control sample: Take a vial of vaccine of the same type and batch number as the vaccine you want to test, and made by the same manufacturer. Freeze the vial until the contents are solid, and then let it thaw. This vial is the control sample. Clearly mark the vial so that it cannot later be used by mistake. |
| (2) | Choose a test sample: Take a vial of vaccine from the batch that you suspect has been frozen. This is the test sample. |
| (3) | Shake the control and test samples: Hold the control sample and the test sample together in one hand and shake vigorously for 10-15 seconds. |
| (4) | Allow to rest: Leave both vials to rest. |
Compare the vials: View both vials against the light to compare the sedimentation rate. If the test sample shows a much slower sedimentation rate than the control sample, the test sample is probably potent and may be used. If the sedimentation rate is similar and the test sample contains flakes, the vial under test has probably been damaged by freezing and should not be used. Note that some vials have large labels which conceal the vial contents. This makes it difficult to see the sedimentation process. In such cases, turn the sample and reference vials upside down and observe sedimentation taking place in the neck of the vial.
Subsequent action: If the test procedure indicates that the test sample has been damaged by freezing, you should notify your supervisor immediately. Standard Operating Procedures should then be followed to ensure that all damaged vaccine is identified and that none of this damaged vaccine is distributed or used.
Table 1 depicts the official schedule for the Expanded Programme on Immunisation in South Africa

TABLE 1 : RECOMMENDED CHILDHOOD IMMUNISATION SCHEDULE
|
When should a child be immunised? |
||
|
Age of Child |
Which Vaccine? |
How and where is it given? |
|
At birth |
BCG (vaccine against tuberculosis) Polio vaccine |
Intradermal injection in upper right arm Drops by mouth |
|
6 weeks old |
Polio vaccine DTP2 vaccine Hib3 vaccine Hepatitis B vaccine |
Drops by mouth Injection in left thigh Injection in left thigh Injection in left thigh |
|
10 weeks old
|
Polio vaccine DTP4 vaccine Hib5 vaccine Hepatitis B vaccine |
Drops by mouth Injection in left thigh Injection in left thigh Injection in left thigh |
|
14 weeks old |
Polio vaccine DTP6 vaccine Hib7 vaccine Hepatitis B vaccine |
Drops by mouth Injection in left thigh Injection in left thigh Injection in right thigh |
|
9 months old |
Measles vaccine |
Injection in left thigh |
|
18 months old |
Polio vaccine DTP7 vaccine Measles vaccine |
Drops by mouth Injection in left thigh Injection in left thigh |
|
5 years old |
Polio vaccine DTP9 vaccine |
Drops by mouth Injection in left thigh |
2 vaccine against diphtheria, whooping cough and tetanus (lock-jaw)
3 vaccine against Haemophilus influenzae type b
4 vaccine against diphtheria, whooping cough and tetanus (lock-jaw)
5 vaccine against Haemophilus influenzae type b
6 vaccine against diphtheria, whooping cough and tetanus (lock-jaw)
7 vaccine against Haemophilus influenzae type b
8 vaccine against diphtheria, whooping cough and tetanus (lock-jaw)
9 vaccine against diphtheria and tetanus only