| 1.2.1 |
Appearance of pharmacy premises |
| (a) |
The design and layout of the pharmacy must permit a logical flow of work, effective communication and supervision and ensure effective cleaning and maintenance and must minimise the risk of errors, cross-contamination and anything else which would have an adverse effect on the quality of products. |
| (b) |
All parts of the premises must be maintained in an orderly and tidy condition. |
| (c) |
The name of the responsible pharmacist must be displayed conspicuously over the main entrance of a pharmacy. |
| (d) |
The name and surname of the pharmacist(s) on duty must be displayed conspicuously in or outside the pharmacy for purposes of identification of such person(s) by the public. |
[Rule 1.2.1 (d) substituted by Board Notice 50, GG 38511, dated 27 February 2015]
| (e) |
The pharmacist(s) and pharmacy support personnel on duty must wear a name tag or badge indicating his/her name, surname and registered designation (e.g. responsible pharmacist, specialist, etc.) for the purposes of identification of such person by the public. This may be combined into a single badge or two separate badges. |
[Rule 1.2.1 (e) substituted by Board Notice 50, GG 38511, dated 27 February 2015]
| (f) |
The external appearance of pharmacies must inspire confidence in the nature of the health care service that is provided and portray a professional image. |
| (g) |
Entrances, dispensing counters and doorways must be accessible to disabled persons. |
| 1.2.2 |
Another business or practice in a pharmacy or a pharmacy in another business |
| (a) |
the owner and/or the responsible pharmacist of a pharmacy must obtain the approval of Council prior to allowing a person who is not registered with Council to conduct a separate business or practice in the pharmacy. |
| (b) |
the following will be considered another business or practice in a pharmacy; if the: |
| (i) |
business or practice derives income or profit from members of the public for the activities, services or products provided; |
| (ii) |
transactions arising from the activities, services or products provided do not form part of the pharmacy records; |
| (iii) |
customer records held by the other business or practice are separate from the pharmacy records; |
| (iv) |
health professional or person providing services in the business or practice is not employed by the pharmacy; |
| (v) |
business or practice providing services in the pharmacy has its own title, branding and/or is traditionally known by the public as such; and |
| (vi) |
business or practice operating within the pharmacy is owned by a third party. |
[Rule 1.2.2 substituted by Board Notice 70, GG44822, dated 9 July 2021]
| 1.2.2.1 |
Another business or practice in a pharmacy |
The following will be applied by Council in considering applications for another business or practice in a pharmacy. The operation of another business or practice, within a pharmacy must be such that:
| (a) |
the other business or practice does not pose any conflicting interest either ethically or professionally to the practice of pharmacy such as: |
| (i) |
compromise the pharmacy as a health establishment; |
| (ii) |
add any security risk to the acquisition, keeping and supply of medicines; |
| (iii) |
add risk to the patients, particularly in terms of patient confidentiality and the right to privacy; |
| (iv) |
compromise the quality, safety, and efficacy of the medicine; and/or |
| (v) |
compromise the image of the pharmacy and the profession in general. |
| (b) |
the activities of the other business or practice must not interfere or compromise the operations of the pharmacy; |
| (c) |
the area of the business or practice must be clearly identified, permanent and visibly demarcated within the pharmacy; |
| (d) |
the other business or practice shall not operate outside the operating hours of the pharmacy, if the other business or practice shares the same entrance as the pharmacy; and |
| (e) |
the other business or practice may not sell products that are prohibited from being sold in a pharmacy, as per rule 2.29. |
[Rule 1.2.2.1 substituted by Board Notice 70, GG44822, dated 9 July 2021]
| 1.2.2.2 |
A pharmacy in another business |
Where a pharmacy is situated within another business which is not a hospital or other health establishment—
| (a) |
The location of the pharmacy within another business shall take into consideration the: |
| (i) |
accessibility of pharmaceutical services; |
| (ii) |
security aspects relating to the acquisition, storing and supplying of medicines; |
| (iii) |
risk relating to patients, particularly in terms of patients’ confidentiality and the rights to privacy; |
| (iv) |
quality, safety and efficacy of medicines is not compromised; |
| (v) |
pharmacy as a health establishment is not compromised; and |
| (vi) |
the pharmacy as a health establishment and the profession in general is not compromised. |
| (b) |
The pharmacy premises must be clearly identified, permanent and visibly demarcated from the premises of any other business; |
| (c) |
For the purpose of protecting access to medicines and patient confidentiality, such areas (dispensary and where medicines and patient records are kept) must be secured and closed off; and |
| (d) |
In order to comply with the requirement of accessibility to pharmaceutical services, a pharmacist must have 24-hour access to the pharmacy. |
[Rule 1.2.2.2 substituted by Board Notice 70, GG44822, dated 9 July 2021]
| 1.2.3 |
Security in pharmacy premises |
| (a) |
Careful consideration must be given to the overall security of the pharmacy. It must be lockable and as far as possible exclude any unauthorised entry. |
| (b) |
A security policy must be implemented which is designed to ensure as far as possible the safety of both staff and medicines, and must take account of local crime prevention advice. |
| 1.2.4 |
Control of access to pharmacy premises |
| (a) |
The responsible pharmacist of a pharmacy must ensure that every key, key card or other device, or the combination of any device, which allows access to a pharmacy when it is locked, is kept only on his/her person or the person of another pharmacist at all times. |
| (b) |
Control of access to pharmacy premises, which would include the design and layout of the pharmacy, must be of such a nature that only registered pharmacy personnel have direct access to medicine. |
| (c) |
A procedure must be in place to ensure access to pharmacy premises in an emergency situation in compliance with the Occupational Health and Safety Act 85 of 1993. |
| 1.2.5 |
Safety of pharmacy premises |
| (a) |
Working conditions must be arranged to protect the safety of the public and people working on the premises and comply with relevant legislation relating to safety in the workplace. |
| (b) |
There must be a fire extinguisher in the pharmacy which must be checked regularly |
| (c) |
All staff must be familiar with the fire procedure. |
| (d) |
Electrical equipment must be safe and maintained regularly. |
| (e) |
Proper provision must be made for an adequate number of electrical sockets. Care must be taken to avoid trailing wires across floors, work surfaces or sinks. |
| (f) |
A health and safety procedure must be available and read and signed by all staff. |
| 1.2.6 |
Condition of pharmacy premises |
| (a) |
The walls, floors, windows, ceiling, woodwork and all other parts of the premises must: |
| (ii) |
be kept in such good order, repair and condition as to enable them to be effectively cleaned and to prevent, as far as is reasonably practicable, any risk of infestation. |
| (b) |
Countertops, shelves and walls must be finished in a smooth, washable and impermeable material which is easy to maintain in a hygienic condition. |
| 1.2.7 |
Construction of pharmacy premises |
Construction of the premises must ensure that the entry of insects, animals (especially rodents) or birds is prevented and that the premises can be easily cleaned and disinfected.
| 1.2.8 |
Environment in pharmacy premises |
| (a) |
Storage of thermolabile medicines must be in accordance with the storage instructions of the manufacturer. |
| (b) |
Refrigerators used for the storage of thermolabile medicine must be calibrated regularly. |
| (c) |
Products must be protected from the adverse effects of light, freezing or other temperature extremes and dampness. |
| (d) |
Levels of heat, light, noise, ventilation, etc., must exert no adverse effects on pharmaceutical stock as well as personnel. |
| (e) |
All parts of the premises must have suitable and effective means of heating or cooling, lighting and ventilation. If windows can be opened, they must be locked securely when the pharmacy is closed. |
| (f) |
Background music or other broadcasts in the pharmacy must not be played at such a volume so as to cause distraction. |
| 1.2.9 |
Hygiene in pharmacy premises |
| (a) |
There must be an area where equipment and other utensils can be washed which has a source of hot and cold tap water. |
| (b) |
There must be a suitable, clean wash hand basin made of a smooth, washable and impermeable material which is easy to maintain in a hygienic condition and has a source of hot and cold tap water and a closed drainage system. |
| (c) |
Toilet facilities must be kept clean and in good order. |
| (d) |
Hand-washing facilities must be provided in the toilet area or the lobby together with a conspicuous notice requesting users to wash their hands. Facilities must include readily available hot water, soap and clean towels or other satisfactory means of drying the hands. |
| (e) |
Toilet areas must not be used for storage, or as a source of water for dispensing. |
| 1.2.10 |
Storage areas in pharmacy premises |
| (a) |
Storage areas must have sufficient shelving constructed from a smooth, washable and impermeable material, which is easy to maintain in a hygienic condition for the keeping of medicines above floor level. |
| (b) |
Storage areas for Pharmaceuticals must be self-contained and secure. |
| (c) |
Storage areas must be large enough to allow for orderly arrangement of stock and proper stock rotation. |
| 1.2.11.1 |
Size of dispensary |
| (a) |
The size of the dispensary must reflect the volume of prescriptions dispensed and allow a safe and efficient flow of work and effective communication and supervision for the number of persons working in the dispensary. |
| (b) |
The minimum area for the dispensary necessary to allow a safe and efficient flow of work and effective communication and supervision, will depend on a number of factors, which include the number of prescriptions dispensed, the daily pattern of prescription peaks, the configuration of available space and the space available elsewhere in the premises for storage of stock. |
| (c) |
Dispensaries in new pharmacies must be designed to accommodate the forecast workload and the maximum available space must be allocated. In existing pharmacies space requirements must be monitored on a continuing basis. |
| 1.2.11.2 |
Suitability of dispensary |
| (a) |
The dispensary, its fittings and equipment must be adequate and suitable for the purpose of dispensing. |
| (b) |
The dispensary surface area must be sufficient for the volume of prescriptions dispensed. A clear working surface area of at least 90 cm by 1 m must be provided for each pharmacist or other person registered with Council who works in the dispensary. |
| (c) |
The temperature in the dispensing area must be maintained below 25°C and there must be an air-conditioner installed in the dispensary which is in good working order. |
| (a) |
A suitable and adequate means of waste disposal must be available and in use. |
| (b) |
Waste material must not be allowed to accumulate and must be collected in suitable covered (as applicable) receptacles for removal to collection points. |
| (c) |
Written sanitation procedures must be available detailing schedules, methods, materials and equipment available. Responsibility must be assigned in writing. |
| (d) |
Under no circumstances must substances be disposed of down surface water drains, e.g. storm water drains. |
| (e) |
In all situations, a pharmacist must use his pharmaceutical knowledge and skill, together with any necessary expert advice from a Local Authority/Provincial Department of Health, to segregate and dispose of materials, and bio-medical waste safely and in accordance with regulations. |
| 1.2.11.4 |
Dispensing equipment |
There must be adequate, suitable dispensing equipment in the dispensary. Each item must be clean, in good repair and of suitable material. The list below provides a standard. Equipment must be specific for each service which may be provided in the pharmacy:
| (a) |
A suitable means of counting tablets and capsules. This equipment must be cleaned regularly so that cross-contamination between products is avoided. |
| (b) |
An accurate dispensing balance with proof of annual maintenance (if compounding is performed in the pharmacy). |
| (c) |
A range of graduated, stamped glass or plastic measures. |
| (d) |
A refrigerator equipped with a suitable thermometer and capable of storing products at temperatures between 2°C and 8°C. The refrigerator must be cleaned, defrosted and checked periodically to ensure efficient running. This refrigerator must be used only for storing pharmaceutical products. Where applicable, a freezer for the storage of polio and measles vaccines and ice packs must be available. |
| (e) |
A suitable range of dispensing containers for medicinal products. The use of child-resistant closures is encouraged. |
| (f) |
Dispensing labels. Additional warning labels must be available, unless those warnings are printed on the dispensing labels. Where computer software is relied on for warnings/interactions, this must be the latest version available. |
| (g) |
An ointment tile and spatula, stirring rods, pestles, mortars and other appropriate equipment (if compounding is performed in the pharmacy). |
| (h) |
Where sterile or aseptic dispensing is to be carried out in accordance with the Medicines Act, suitable facilities must be available. |
| (i) |
Where cytotoxic or other hazardous substance preparation is to be carried out in accordance with the Medicines Act, a suitable cabinet that complies with generally accepted GMP standards must be provided. |
| (j) |
Suitable means for sterilisation of medicinal products if products that require sterilisation are prepared in the pharmacy. |
| (k) |
Suitable refuse receptacles. |
| 1.2.11.5 |
Reference sources |
The following reference material in the current editions (unless otherwise indicated) must be available for consultation in all pharmacies:
| (a) |
one of the last five (5) editions of Martindale. |
[Rule 1.2.11.5 (a) substituted by Board Notice 35, GG 35095, dated 2 March 2012]
| (c) |
Daily Drug Use (Tincture Press Publications) or other drug interactions reference source; |
| (e) |
a comprehensive textbook on Pharmacology; |
| (f) |
a medical dictionary; |
| (g) |
the latest copy of the Pharmacy Act 53 of 1974, as amended, and the Medicines and Related Substances Act 101 of 1965, as amended; |
| (h) |
a Good Pharmacy Practice Manual; |
| (i) |
a comprehensive textbook on complementary medicine (where applicable); |
| (j) |
a Paediatric Dosing Reference Guide (where applicable); |
| (k) |
Pharmacopoeia (BP, USP, EP) (where applicable); |
| (l) |
the latest copy of the Standard Treatment Guidelines and Essential Drug List for the appropriate level of care. |
A wider range of reference material should be available in institutional pharmacies and in premises approved for pre-registration training. Electronic access to the required references is also acceptable.
A responsible pharmacist may in exceptional circumstances apply to Council for a relaxation of the minimum requirements relating to reference books.
| (a) |
A waiting area(s), which is under cover, must be situated near the dispensing area, areas for counselling and the furnishing of information and/or consultation areas. |
| (b) |
Comfortable seating must be provided. |
| (c) |
The amount of seating available in the waiting area must depend on the number of patients expected to arrive in/at the pharmacy any one time. |
| (d) |
Appropriate health-related literature must be provided. |
| 1.2.13 |
Areas for counselling and the furnishing of advice |
A minimum standard for pharmacies where medicines are supplied directly to the public is a suitable area for the furnishing of advice to patients in a reasonably private environment. The following minimum standards apply:
| (a) |
The area(s) must be easily accessible and, where possible, be close to the dispensary. |
| (b) |
Clear directions must be provided if the area is not close to the dispensary. |
| (c) |
The area(s) must be constructed in such a manner as to ensure reasonable privacy to the patient at all times and eliminate background noise as far as possible. |
| (d) |
The area(s) must have a professional appearance and be kept clean and tidy. |
| (e) |
The area(s) must have sufficient space to promote appropriate counselling and demonstration of the correct and safe use of specific medicines as required. |
| 1.2.13.1 |
Types of areas for the furnishing of information and advice and the counselling of patients |
Three types of areas for the furnishing of information and advice should be considered for the pharmacy, depending on the services offered by the pharmacy and the degree of privacy required. These models are:
| (a) |
semi-private area at each point where dispensing of medicine to the patient or the patient's agent/caregiver occurs; |
| (c) |
a consultation area for the provision of screening and monitoring services. |
Every pharmacy must have at least one type of area for the furnishing of information and advice. In cases where a pharmacy only has a semi-private area(s) at each point where dispensing of medicine to the patient or the patient's agent/caregiver occurs, there must in addition be access to another separate private room/area where communication can take place between a pharmacist and a patient or the patient's agent/caregiver in private.
Standards for the different types of areas, including the equipment required, are provided below.
[Rule 1.2.13.1 substituted by Board Notice 83, GG 31378, dated 29 August 2008]
| 1.2.13.2 |
Semi-private area |
Semi-private area(s)
This area(s) is for the provision of information and/or advice that may occur in an area visible to other patients.
| (a) |
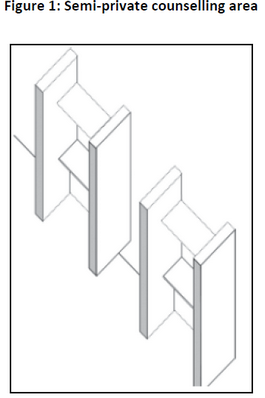
The area(s) could be of a modular 'bank teller' type, where a counter is utilised, offering the patient or his/her agent/caregiver reasonably private access to the pharmacist. Another option is an aperture, which is surrounded by a 'telephone booth' type structure to prevent other persons from crowding around the patient or his/her agent/caregiver who is communicating with the pharmacist. Figure 1 is a schematic representation of an example of a semi-private area. |
| (b) |
In such an area(s), patient counselling may take place in a professional manner regarding medicine use and other relevant information, but does not provide the privacy required to advise patients on sensitive issues. |
[Rule 1.2.13.2 substituted by Board Notice 83, GG 31378, dated 29 August 2008]
| 1.2.13.3 |
Separate private area |
The area envisaged in this model is a small private room within the pharmacy, which is in close proximity to the dispensary.
| (a) |
The area should be professionally planned, furnished and equipped, so as to allow the pharmacist to consult and counsel patients who may have sensitive emotional or health care problems and advise a patient and/or his/her agent/caregiver on medicines, and other related issues. |
[Rule 1.2.13.3(a) substituted by Board Notice 83, GG 31378, dated 29 August 2008]
| (b) |
The area should be such that the pharmacist has easy access to the area from the dispensary, and the patient easy access from the waiting area. |
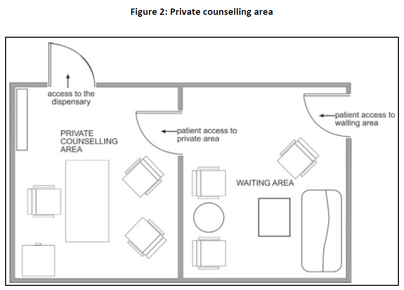
| (c) |
The size of the area should be adequate. It should have a table, comfortable chairs and shelves for reference books. The pharmacist's qualifications could also be displayed. Informative wall posters and charts could be used. Figure 2 is a representation of an example of a private area for the furnishing of advice. |
[Rule 1.2.13.3(c) substituted by Board Notice 83, GG 31378, dated 29 August 2008]
| 1.2.13.4 |
Consultation area for the provision of screening and monitoring services |
A consultation area for the provision of screening and monitoring services is an isolated area within which the pharmacist can consult in private with a patient and/or perform certain screening and monitoring services, e.g. cholesterol tests or blood pressure monitoring.
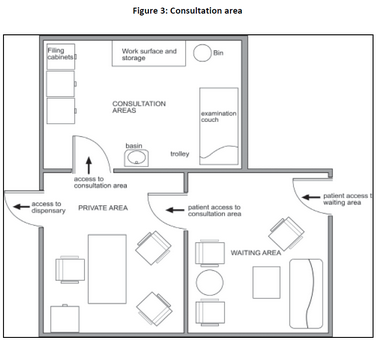
| (a) |
The consultation area must have a professional appearance and must be kept clean and tidy. Figure 3 is a schematic representation of such a consultation area. |
| (b) |
The consultation area should have sufficient space (at least 7.5 square meters). |
[Rule 1.2.13.4.1(b) substituted by Board Notice 83, GG 31378, dated 29 August 2008]
| (c) |
The working surface in the area must be of impermeable washable material. |
| (d) |
The area must at least have the following: |
| (i) |
an examination couch with spare clean sheets; |
| (ii) |
a suitable trolley and/or cabinet for the necessary equipment; |
| (iv) |
applicable facilities for the taking and analysis of urine and/or blood samples where necessary; |
| (v) |
a wash hand basin with hot and cold running water; |
| (vi) |
a closable rubbish bin with a lid and disposable plastic liners; |
| (vii) |
effective equipment for record keeping; and |
| (viii) |
a biohazardous materials bin and sharps container. |
| (e) |
It is advisable to have a refrigerator with a freezing compartment in the consultation area, especially when immunisation services are provided. |
| (f) |
A toilet in the vicinity of the consultation area is strongly recommended. |
| (g) |
A comfortable waiting area for patients situated, if possible, near the consultation area is a necessity. |
| (h) |
Procedures must be in place to ensure that medicines and working areas are not contaminated by infected materials and/or instruments. |
The services rendered would determine the minimum equipment needed. The following list may be used as a guideline:
| (a) |
a sphygmomanometer (Mercury type) and stethoscope; |
| (d) |
a snellen chart (E-type); |
| (e) |
a blood glucose meter; |
| (g) |
a 'Road to Health Card' and additional copies for supply to patients; |
| (i) |
equipment used for first aid, e.g. plaster, gauze, cotton wool, sterile wound dressings, scissors; |
| (j) |
disposable rubber gloves; |
| (k) |
oxygen with a flow meter; |
| (l) |
a clinical thermometer; |
| (m) |
alcohol swabs of 70% alcohol solution or surgical spirits; |
| (p) |
towels or paper towels; |
| (q) |
disinfectants (especially disinfectants recommended for control of the AIDS virus e.g. 2% gluteraldehyde, 1% halocide, sodium hydrochloride, biodecyl 1%) for equipment and clothing/fabric, ready prepared; |
| (r) |
applicable equipment for approved screening tests, should these services be rendered; |
| (t) |
a scale plus baby scale; and |
| (u) |
a height chart and tape measure. |
| 1.2.14 |
Pharmacy transactions |
For a pharmacy that is within another business, the following transactions shall take place within the pharmacy only:
| (a) |
The prescription shall be handed over or delivered to a designated pharmacy staff or submitted to the pharmacy directly, e.g. by electronic transmission, postal/courier delivery or deposited in a locked prescription receiving box which is only accessible to the pharmacist (as per GPP rules); |
| (b) |
the dispensing process (phase 1, 2, and 3); |
| (c) |
the recording of medicines and scheduled substances, which includes the recording of schedule 1 and 2 medicines in terms of Regulation 11 of the General Regulations to the Medicines and Related Substance Act 101 of 1965; |
| (d) |
the payment for all medicine and scheduled substances. |
[Rule 1.2.14 inserted by Board Notice 35, GG 35095, dated 2 March 2012]
 Public Service Commission Act, 1997
Public Service Commission Act, 1997


