BACKGROUND
Sexual and reproductive health is fundamental for the overall health and well-being of individuals, couples and families, and for the social and economic development of communities and countries. This standard will regulate the following services as provided by pharmacists: HIV testing, Pharmacist-Initiated Management of Antiretroviral Therapy (PIMART), emergency postcoital contraception (EPC), and family planning including hormonal contraception.
South Africa has the highest prevalence of HIV in the world. Sexual and reproductive healthcare constitutes a significant portion of pharmacists’ daily practice, especially in the primary healthcare setting. Pharmacists have a professional and social responsibility to educate the public on sexual and reproductive health matters. They should also contribute to efforts aimed at the prevention and treatment of sexually transmitted infections (STIs), including HIV/AIDS, and the prevention of unplanned pregnancies. Pharmacists are well placed to provide therapeutic guidance to women of all ages, able to advise on contraception, fertility, pregnancy and menopause. Individuals seeking access to abortion services, and those who fall victim to sexual and gender-based violence can seek confidential and professional assistance from the pharmacist.
Pharmacists are ideally suited to assist in addressing these needs since they are readily available and easily accessible. Given the broad extent of pharmaceutical services provided in pharmacy, the setting can also be considered to be non-stigmatising.
It is recommended that the following package of services be available to all individuals requesting access to sexual health and related services:
| (a) |
ART initiation for those diagnosed with HIV |
| (b) |
Condoms and lubricants |
| (d) |
Emergency post-coital contraception (EPC) |
| (e) |
Fertility screening and referral |
| (g) |
Hormonal and non-hormonal contraception |
| (h) |
Menopause/end of reproductive years (transition phase from contraception to possible Post-Menopausal Hormone Replacement Therapy (PMHRT)) |
| (i) |
Post-exposure prophylaxis (PEP) |
| (j) |
Pre-exposure prophylaxis (PrEP) |
| (l) |
Syndromic STI diagnosis and treatment |
The purpose of this standard is to provide the minimum standard in the provision of sexual health services by pharmacists who are appropriately trained and authorised to provide such services and includes HIV testing, PIMART, EPC and family planning and hormonal contraception services.
“AIDS” Acquired Immunodeficiency Syndrome
“ART” Antiretroviral Therapy is the use of antiretroviral (ARV) medicines to achieve viral suppression and which are prescribed for life
“CHC” Community Health Centre
“EPC” Emergency Post-coital Contraception refers to a method of hormonal contraception that may be used after sexual intercourse to prevent pregnancy
“Face to face/in person” means where the patient and the pharmacist are physically in the same place at the same time
“HIV” Human Immunodeficiency Virus
“Informed consent” means permission granted to the pharmacist who provides the requested service by a person requiring such a service in full knowledge of the procedures, possible consequences, risks, benefits and costs of such service
“PEP” Post-Exposure Prophylaxis; means ARV medication taken to prevent HIV after a possible exposure and which is initiated within 72 hours after a possible exposure to HIV
“PHC” Primary Health Care Clinics defined in the Regulations relating to the practice of pharmacy
“PIMART” Pharmacist-Initiated Management Antiretroviral Therapy and, for purposes of this standard, includes PEP, PrEP and ART
“PMHRT” Post-Menopausal Hormone Replacement Therapy
“PrEP” Pre-Exposure Prophylaxis; is medicine which is taken by individuals at risk for acquiring HIV infection, to prevent becoming infected with HIV from sex or injection drug use
“Pre-test counselling” means counselling given to an individual before a diagnostic test to ensure that a person requiring the service has sufficient information in order to make an informed decision regarding the performance of such a test
“Post-test counselling” means the counselling provided to the tested person upon making the test result known to them
“RHC” Reproductive Health Clinic
“SOP” Standard Operating Procedure
“STI” Sexually Transmitted Infection
“Telepharmacy” means a pharmacist – patient real-time interaction (delivering pharmaceutical care) via audio-visual means of communication
“WHO” World Health Organisation
Pharmacists who provide any of the services identified in this standard should: (a) ensure that they have adequate training, knowledge and skills to provide the services which they offer; (b) ensure that they are knowledgeable with respect to the latest developments in sexual and reproductive healthcare services through self-training, training by accredited providers and/or continuing professional development courses; and (c) ascertain themselves of the instructions and requirements of all the products that are used in the provision of the respective services they offer.
| 3.2 |
Physical facilities and equipment |
| (a) |
All services contained in this standard shall be provided in a private consultation area in the pharmacy which complies with the minimum requirements as stipulated in Rule 1.2.13 - Areas for counselling and the furnishing of advice. The following additional equipment and fittings must be available in the consultation area, where applicable: |
| (i) |
A separate fridge for the storage of test materials, blood samples and other biohazardous materials that may be collected during the rendering of any of the services. |
| 3.3 |
Counselling and confidentiality |
| (a) |
It is critical to establish a trust relationship with a person requiring the services detailed in this standard. Such a person deserves the full attention of the pharmacist they are consulting with, including comprehensive examination and counselling with adequate record keeping. Therefore, complete and uninterrupted privacy must be maintained during the provision of these services. |
| (b) |
Patient information must only be disclosed to another duly authorised pharmacist or to a person authorised by law to request it with the consent of the patient. |
| (c) |
Based on the service provided, a person requiring the service must be supplied with the relevant patient information leaflets that conform to Regulation 12 of the General Regulations (2017), published in terms of the Medicines and Related Substances Act, 101 of 1965. A person to whom such services were rendered, should be encouraged to contact the pharmacist if he/she has any further questions. |
| (d) |
During testing and counselling, the pharmacist must act in an ethical and professional manner that takes into consideration the fundamental personal constitutional rights of clients. |
| (e) |
If the pharmacist becomes aware that a child has been physically harmed, sexually abused, assaulted or exploited by another person, the pharmacist must report these circumstances to the appropriate local authority. |
| (a) |
A person requiring any of these services, must provide informed consent which clearly stipulates the nature of the service(s) to be rendered. |
| (b) |
In terms of Section 129 of the Children’s Act, 38 of 2005, a child over the age of 12 years may provide consent to the services identified herein, provided such child is of sufficient maturity and decisional capacity to understand the various implications of the treatment including the risks and benefits thereof. |
| 3.5 |
Documentation and record keeping |
| (a) |
All services must be provided with the required consultation, written consent and written and/or electronic completion of the patient medical history file. |
| (b) |
Patient information may only be disclosed to another duly authorised pharmacist or healthcare professional, or to a person authorised by law to request it with the consent of the patient. |
| (c) |
Where applicable, test results must be provided to the person who required the service/s, in writing. |
| (d) |
All referral documents must be signed by the pharmacist who rendered the service. |
| (e) |
The name of the pharmacy, as well as the name of the pharmacist who performed the test, must appear on all applicable records and documentation. |
| (f) |
Consultations, discussions and any relevant test results must be conducted with, and communicated to, the patient. |
| (g) |
The following information pertaining to a person who required the service must be kept in either hard copy or electronic format for a period of not less than five (5) years: |
| (i) |
Name, address and ID number of the patient; |
| (iii) |
Applicable medical and medicine history; and |
| (iv) |
Any further applicable and required records pertaining to the respective services as regulated by this minimum standard. |
| 3.6 |
Professional and ethical responsibility |
| (a) |
A pharmacist who provides any of the services as regulated by these minimum standards shall: |
| (i) |
remain objective and refrain from personal judgment or indicating disapproval towards the patient or any person accompanying the patient; and |
| (ii) |
at all times show respect in terms of population diversity and personal beliefs, and promote effective patient-pharmacist communication during and following consultations. |
| (b) |
A pharmacist who provides any of the services as regulated by these minimum standards shall bear in mind that patients seeking access to one or more of these services may do so for various reasons, which may include inter alia: |
| (i) |
fear of becoming pregnant; |
| (ii) |
embarrassment at failing to use contraceptives effectively; |
| (iii) |
lack of knowledge about EPC; |
| (iv) |
worry about missing the narrow window of opportunity for EPC; |
| (v) |
general embarrassment about sexual health matters; |
| (vi) |
rape and/or sexual abuse trauma; and |
| (vii) |
concern about HIV/AIDS and/or sexually transmitted infections (STIs). |
| (c) |
Notwithstanding appropriate training and being duly authorised, pharmacists who do not wish to provide any of the services due to personal reasons should always remain objective, professional and non-judgmental when addressing and communicating with a patient. In such cases, the person requiring the relevant service/s should be referred to an alternative health care worker or facility where they can gain access to the service they may require. Such referrals may include: |
| (i) |
another duly authorised pharmacist in the same pharmacy; |
| (ii) |
another pharmacy in the vicinity; |
| (iii) |
a medical practitioner; and/or |
| (iv) |
a nearby hospital, Community Health Clinic, Primary Health Clinic or Rural Health Clinic. |
| (d) |
If questioned regarding the reason for not providing the requested service, the pharmacist should answer in a manner that is not stigmatising, opinionated or biased and that will not engender a sense of unease in a person requiring the service. |
| (e) |
In the provision of any of these services, the pharmacist must act to the best of their ability and in the best interest of the patient. |
| (f) |
Professional cooperation between local pathology laboratories and other healthcare professionals and facilities should as far as possible be fostered and maintained. |
| (g) |
All pharmacy personnel should be informed of the services which are available at the pharmacy. Personnel must be appropriately trained to approach and interact with patients or persons requiring any of the services in an appropriate manner. |
| 3.7 |
Standard operating procedures (SOPs) |
| (a) |
A pharmacist providing any of these services must ensure that written policies and standard operating procedures are in place for each of the respective services that are provided. |
| 3.8 |
Advertising of the services |
| (a) |
Any of these services may be advertised in a manner that is consistent with the Rules relating to acts or omissions for which the Council may take disciplinary action and the Rules relating to the code of conduct of pharmacists and other registered persons. All advertisements must be factually correct, must not be misleading and must not harm the dignity of the profession. |
| 4. |
PERFORMING HIV TESTING |
| 4.1 |
Introduction There are three types of tests available: nucleic acid tests (NAT), antigen/antibody tests, and antibody tests. A NAT looks for the actual virus in the blood and involves drawing blood from a vein. Tests to detect HIV1 and/or HIV2 antibodies are now widely used to diagnose HIV infection. These rapid HIV screening tests are based on immunochromatography or immunofiltration which identify HIV infection based on their selective interaction with and recognition of HIV-targeting antibodies. Due to its low cost, robust efficacy, and ease of use, rapid HIV screening tests are useful for the first-line and early detection of HIV infection across a broad range of settings, including pharmacies. The following minimum standard should be observed when pharmacists provide this service. |
| 4.2 |
Training In addition to the requirements stated in Rule 3.1 -Training above, pharmacists providing an HIV testing service must: |
| (a) |
undergo training that equips them with the knowledge and skills to perform HIV antibody tests, interpret the results and counsel a person requiring this service. |
| (b) |
pharmacists who provide this service should demonstrate knowledge of: |
| (i) |
the immune system and the biology of the virus; |
| (ii) |
signs and symptoms of HIV and AIDS; |
| (iii) |
opportunistic infections that are often comorbid with HIV and AIDS; |
| (iv) |
other diseases that are often associated with AIDS; |
| (v) |
the therapeutic principles of HIV medicine therapy; |
| (vi) |
modes of HIV transmission; |
| (vii) |
the practical, ethical, and legal aspects of HIV counselling; and |
| (viii) |
the role of the pharmacist in the prevention of HIV and AIDS. |
| 4.3 |
Pre-HIV test counselling |
| (a) |
HIV testing should always be provided with due consideration to the personal and emotional factors that may be involved in the decision to test and the way the test result is communicated. Therefore, a person requiring this service must always be counselled before being tested for HIV in order to prepare them for the outcome. |
| (b) |
Including the requirements stated in Rule 3.3 - Counselling and confidentiality, pre-HIV-test counselling must include: |
| (i) |
the definition of HIV and AIDS; |
| (ii) |
what an HIV test is and what the purpose of the test is; |
| (iii) |
the nature of the specific test and procedure to be followed and when a result can be expected; |
| (iv) |
the concept of the window period; |
| (v) |
test accuracy and the need for follow-up |
| (vi) |
factors relating to the transmission of HIV infection; |
| (vii) |
the meaning and implications of a negative HIV test result; |
| (viii) |
the meaning of a positive result, including the practical implications that such a result may have, regarding future medical treatment and care, sexual relations, psycho-social implications and occupational routine; |
| (ix) |
assessment of personal risk of HIV infection; |
| (x) |
safer sex and strategies to reduce the risk of transmission; |
| (xi) |
potential perspectives on negative and positive test results; |
| (xii) |
strategies to cope with a positive test result, including discussions on who to inform and how to address personal needs and identify support services; |
| (xiii) |
available social support structures; and |
| (xiv) |
providing a person requiring the service a final opportunity for decision-making about taking the HIV test. |
Including the requirements stated in Rule 3.4 – Informed consent above, informed consent with respect to HIV testing, should include:
| (a) |
HIV testing is not covered by the standard contractual informed consent agreement. |
| (b) |
With respect to HIV testing, informed consent implies that, following the pre-test counselling process, which must be done in a clear and understandable manner, a person requiring the service, understands the following: |
| (i) |
the reason or purpose for which the test is being performed; |
| (ii) |
the advantages and disadvantages of having their HIV status determined; |
| (iii) |
the personal, emotional and practical influence that the result of the HIV test may have on their perspectives and approaches to future treatment; |
| (iv) |
the testing procedure; |
| (v) |
that they may still decline to be tested; |
| (vi) |
that post-test counselling will be done immediately once the test result is available dependent on the nature of the testing procedure followed; and |
| (vii) |
that post-test counselling is an ongoing process. |
HIV testing should be performed as outlined in the National HIV Testing Services Policy document as published by the National Department of Health (NDoH).
| (a) |
It is recommended that universal precautions be taken with all persons requiring the service, i.e. performing testing as if a person is infected. |
| (b) |
The pharmacist must be familiar with the WHO guidelines for the prevention of HIV and hepatitis. |
| (c) |
Where applicable, the pharmacist providing the service must follow the manufacturer’s instructions for the respective HIV tests, carefully and not act in a manner that may adversely affect the test result in any way. |
| 4.6 |
Interpreting test results |
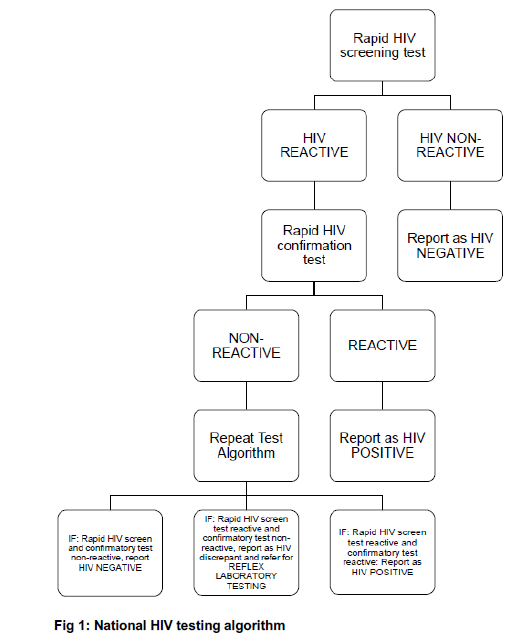
HIV test results may be influenced by a number of factors, including the window period, level of risk, degree of exposure and the number of exposure events, therefore the following should be considered when interpreting test results:
| (a) |
It can take up to 12 weeks for the human body to generate enough HIV antibodies to deliver a positive result. This means that an HIV antibody test may be negative in the early stages after infection may have occurred. This is called the 'window period’ and HIV antibody tests may be negative during this time. |
| (b) |
If the test result is negative and it is suspected that this may be due to the test being performed during the window period, the tested person should be advised to be retested after 12 weeks after being exposed to HIV. |
| 4.7 |
Post-test counselling |
| (a) |
Counselling must be provided to the tested person after the result is known. |
| (b) |
In the case of a negative test result, both during and following a suspected window period, counselling must be provided to the person to ensure that they retest if needed, and that future test results remain negative. |
| (c) |
Post-test counselling in the case of a negative test result should include: |
| (i) |
strategies for reducing the risk of becoming infected with HIV; |
| (ii) |
possibility of infection in the 'window' period; and |
| (iii) |
suggest retesting in three months' time. |
| (d) |
Post-test counselling in the case of a positive test result should include: |
| (i) |
understanding the immediate emotional reaction and concerns of the tested person; |
| (ii) |
the need for confirmatory testing; |
| (iii) |
possible routes of transmission that could have resulted in the test outcome; |
| (iv) |
the difference between being infected and being infectious; |
| (v) |
the importance of notifying sexual partners; |
| (vi) |
the likely progression of infection; |
| (vii) |
implications for the personal, familial and social well-being of the tested person; |
| (viii) |
potential difficulties the tested person may foresee and possible coping strategies to address these; |
| (ix) |
with whom a person may want to share the results with; |
| (x) |
the need for later follow-up supportive counselling and medical care; |
| (xi) |
immediate needs and social support identification; and |
| (xii) |
the availability of care programmes. |
| (e) |
Where applicable, i.e. if continuous counselling, support and care cannot be provided at the testing pharmacy, a person who tested HIV positive must be directed to an appropriate facility, e.g. CHC or PHC, where such services can be provided. If possible, sexual partners and/or families should be encouraged to also undergo counselling. |
| (f) |
It is recommended to establish and foster adequate counselling referral systems in the area of the testing facility, such as liaising with social workers, clinical psychologists and other caregivers in the community. |
| 4.8 |
Documentation and record keeping |
| (a) |
Including the requirements stated in Rule 3.5 - Documentation and record keeping above, the following documentation and record keeping is required: |
| (i) |
patient files should be updated with test results and a summary of the consultation discussed during the pre- and post-testing counselling sessions; and |
| (ii) |
statistics of all pre- and post-test (immediate and ongoing) counselling sessions, as well as the number of HIV tests performed on different persons requiring the service. |
| 5. |
PHARMACIST-INITIATED MANAGEMENT OF ANTIRETROVIRAL THERAPY (PIMART) |
PIMART services are regarded as a necessary and vital mechanism that can be provided to bolster efforts to prevent and treat HIV/AIDS.
In cases where in vitro diagnostic tests are not required, consultations may be offered in person or via telepharmacy.
Including the requirements stated in Rule 3.1 - Training above, pharmacists providing PIMART services are required to undergo supplementary training with an accredited provider registered with Council and obtain a permit issued in terms of Section 22A(15) of the Medicines and Related Substances Act, 101 of 1965.
Including the requirements stated in Rule 3.4 - Informed consent above, informed consent with respect to PIMART services should include:
| (a) |
a thorough understanding of the service and its expected outcome; and |
| (b) |
a counselling process, which is clear and understandable so that a person requiring the service understands the following: |
| (i) |
the reason or purpose for which the service is being provided; |
| (ii) |
the advantages and potential risks of taking antiretroviral medicines for any of the PIMART-related indications; |
| (iii) |
that they may decline the requested service following the counselling process; and |
| (iv) |
the importance of medication adherence and follow-up consultations. |
| 5.4 |
Specific considerations |
For all PIMART services, the following must be observed:
| (a) |
An adequate referral system should be in place to ensure that patients who need clinical oversight will be able to access such services; |
| (b) |
A patient monitoring system that must be used to: |
| (i) |
record the pregnancy and/or HIV status of the person at the time of the person requesting PIMART services; |
| (ii) |
record other required laboratory test results which may be a prerequisite for any of the PIMART services provided; |
| (iii) |
track medication adherence; |
| (iv) |
facilitate the scheduling of follow-up visits; and |
| (v) |
record and track referrals. |
| 6. |
EMERGENCY POST-COITAL CONTRACEPTION (EPC) |
Emergency post-coital contraception (EPC) may be taken by women within a maximum period after having unprotected sex. This period is specific to the method prescribed. In the event that pharmacist-initiated EPC is not indicated, the person should be referred to an appropriate health care provider or facility. Although EPC is not effective if a person requiring the service is already pregnant, EPC is not considered to be harmful to the foetus. EPC is intended for emergency contraceptive purposes only and is not considered to be a suitable contraceptive for repeated use.
| (a) |
Since EPC consists of hormonal medicine intervention, all pharmacists are deemed to have a broad understanding of the available pharmacist-initiated therapy EPC regimens. Including the requirements stated in Rule 3.1 above, pharmacists providing an EPC service must also demonstrate knowledge of: |
| (i) |
the menstrual cycle in terms of hormonal and anatomical change over time; |
| (ii) |
the pharmacology of EPC and its effects on menstrual biology; |
| (iii) |
ovulation, conception and pregnancy; and |
| (iv) |
the different mechanisms of EPC that prevent conception and/or implantation. |
| (a) |
Prior to the dispensing of EPC and in addition to the requirements stipulated in Rule 2.7 - Minimum standards for the dispensing of medicine or scheduled substances on the prescription of an authorised prescriber of the Rules relating to Good Pharmacy Practice - with respect to the dispensing of medicine, the following information must be obtained and signed personally by the person requiring EPC: |
| (i) |
certainty that the patient does not wish to become pregnant; |
| (ii) |
date of the last menstrual cycle of the person to rule out potential current pregnancy; |
| (iii) |
the time that has elapsed since unprotected intercourse occurred; and |
| (iv) |
whether the patient has been a victim of sexual assault. |
| (b) |
Prior to the dispensing of EPC, the person requiring EPC should be counselled with respect to: |
| (i) |
the purpose and intention of EPC; |
| (ii) |
the potential effects that EPC may have on her menstrual cycle and bleeding pattern; |
| (iii) |
current contraceptive use, where applicable; |
| (iv) |
potential risks, other than unwanted pregnancy, involved in having unprotected sex, i.e. STIs; |
| (v) |
the likelihood that EPC may not be effective; |
| (vii) |
what to do in the case of vomiting within two (2) hours after EPC administration; and |
| (viii) |
the benefits of continued, daily oral contraceptives and other methods of contraception. |
| (c) |
In cases where the pharmacist that is providing the service suspects any complication, whether based on the answers obtained on the aspects stipulated in 6.3(a) above or otherwise, a person requiring EPC should be referred to a medical practitioner. |
| (d) |
If the pharmacist becomes aware that a child has been physically harmed, sexually abused, assaulted or exploited by another person, the pharmacist must report these circumstances to the appropriate local authority. |
| (a) |
A verbal request for access to EPC is regarded as consent. |
| (b) |
Under no circumstances may consent for EPC be provided by a person other than the person for whom the EPC is intended. |
| (c) |
EPC may only be dispensed to a person for whom such intervention is intended. |
| 7. |
FAMILY PLANNING (INCLUDING HORMONAL CONTRACEPTION) |
Family planning addresses the ability of individuals and couples to anticipate and attain their desired number of children and the spacing and timing of their births. This can be achieved through various means, including natural pregnancy planning, and the use of hormonal and non-hormonal contraceptive and contragestive methods. Hormonal contraceptives may be supplied in accordance with the provisions of the Medicines and Related Substances Act, 101 of 1965. Further, pharmacists can administer injectable contraceptives if the pharmacist is competent in injection techniques.
| 7.2 |
Specific considerations |
Including the general principles outlined in Rule 3 - General principles above, the following additional requirements pertaining to the provision of family planning services should also be observed:
| (a) |
Pharmacists who wish to provide family planning services must undergo supplementary training from an accredited provider registered with Council and obtain a permit issued in terms of Section 22A(15) of the Medicines and Related Substances Act, 101 of 1965. |
| (b) |
Cooperation must be maintained with local doctors and reproductive health clinics. |
| (c) |
Patients on pharmacist-initiated oral or injectable contraceptives must be referred to a medical practitioner or an authorised RHC at least once a year for a full physical evaluation. |
[Annexure A substituted by insertion of section 2(a) of Board Notice 479, GG49379, dated 29 September 2023: Minimum standards for sexual and reproductive health services proved by pharmacists]
 Businesses Act, 1991 (Act No. 71 of 1991)
Businesses Act, 1991 (Act No. 71 of 1991)
