 Businesses Act, 1991 (Act No. 71 of 1991)
Businesses Act, 1991 (Act No. 71 of 1991)
R 385
Pharmacy Act, 1974 (Act No. 53 of 1974)Board NoticesPrimary Care Drug Therapy Pharmacist (PCDT)Part 3 : Criteria for Accreditation : Primary Care Drug Therapy |
RATIONALE FOR THE PCDT SUPPLEMENTARY TRAINING
The Medicines and Related Substances Act 101 of 1965 (Medicines Act) currently permits a pharmacist to dispense Schedule 1 and 2 medicines without a prescription from an authorised prescriber, as defined in the Act. In striving to develop and utilise pharmaceutical services and the pharmacist's expertise optimally in South Africa, pharmacists who have obtained the Primary Care Drug Therapy (PCDT) supplementary training are issued with a permit in terms of Section 22A(15) of the Medicines Act.
Section 22A(15) “Notwithstanding anything to the contrary contained in this section, the Director General may, after consultation with the South African Pharmacy Council as referred to in section 2 of the Pharmacy Act, 1974 (Act No. 53 of 1974), issue a permit to any person or organisation performing a health service, authorising such person or organisation to acquire, possess, use or supply any specified Schedule 1, Schedule 2, Schedule 3, Schedule 4 or Schedule 5 substance, and such permit shall be subject to such conditions as the Director-general may determine.”
The Section 22A(15) permits are issued by the National Department of Health (NDoH) together with a list (referred to as the PCDT list (Appendix A)) of conditions that the PCDT pharmacist may diagnose and a list of medication (schedule 3, 4 and 5) that the PCDT pharmacist may prescribe and dispense to treat these diagnosed conditions. This PCDT list is in line with the NDoH’s latest Primary Health Care Standard Treatment Guidelines and Essential Medicines List (PHC STG and EML). Only pharmacists who have recorded their PCDT supplementary training with the SAPC may apply for the Section 22A(15) permit for PCDT.
PCDT pharmacists are expected to apply the principles of pharmaceutical care in providing pharmacist-initiated therapy. Pharmaceutical care is patient centred, outcome orientated pharmacy practice that requires the pharmacist to work in conjunction with the patient and the patient's other healthcare providers to promote health, to prevent disease and to assess, monitor, initiate and modify medication use to assure that medicine therapy is safe and effective.
PURPOSE OF THE PCDT SUPPLEMENTARY TRAINING
The purpose of PCDT supplementary training is to equip pharmacists with the essential knowledge in the field of pharmacotherapy and pharmaceutical care and to develop pharmacist’s clinical skills in order to provide pharmacist-initiated therapy within the scope of Good Pharmacy Practice (GPP) rules. PCDT pharmacists must act in the interest of patients and other members of the public and seek to provide the best possible healthcare for the community.
PCDT pharmacists should be able to practise and develop as authorised prescribers to meet the health care needs of the country in line with the PHC STG and EML and as stipulated in the PCDT list, as amended from time to time by the NDoH. They are responsible for their own safe and efficient practice and are bound by GPP rules. Minimum standards, Ethical rules and the Code of Conduct for pharmacists and other persons registered in terms of the Act in accordance with section 35A(b)(i) of the Pharmacy Act, 53 of 1974 and must comply with the Medicines Act.
The goal of PCDT pharmacists is to improve patient care without compromising patient safety, increase access to health care services, optimise the skills of health professionals and contribute to the introduction of a more flexible multidisciplinary primary health care (PHC) team. They are expected to practise in collaboration with other health care professionals and refer patients to other members of a health care team when necessary. PCDT pharmacists must ensure that they behave with integrity and honesty, and do not engage in any behaviour or activity likely to bring the profession into disrepute and undermine public confidence in the profession.
PCDT pharmacists have a professional responsibility to keep themselves abreast of clinical and professional developments. They are expected to keep up to date with evidence and best practices in the management of conditions which they are authorised to treat. They must establish peer to peer interaction and discussion or mentoring arrangements providing an opportunity for reflection on prescribing, as well as other aspects of practice. They may only prescribe medicines for a patient whom they have assessed personally and may not prescribe any medicine for their own use or for any person with whom they have close personal or emotional relationship.
TARGET GROUP FOR PCDT SUPPLEMENTARY TRAINING
Practicing Pharmacists as defined in the Regulations relating to Continuing Professional Development.
MINIMUM ENTRANCE CRITERIA TO THE PCDT SUPPLEMENTARY TRAINING
Pharmacists who wish to enrol for the PCDT supplementary training must:
| • | be in possession of a Bachelor of Pharmacy (BPharm) degree, or recognised equivalent, |
| • | be registered at the SAPC as a Pharmacist, and |
| • | have completed their community service year. |
DURATION AND NQF LEVEL OF PCDT SUPPLEMENTARY TRAINING
The curriculum will be presented at NQF level eight. This supplementary training comprises 1200 notional hours (120 credits) to be completed on a part-time basis, over 24 to 36 months. The training can be provided as multiple modules adding up to the required number of notional hours.
PCDT SUPPLEMENTARY TRAINING RULES
In order to be credited with PCDT supplementary training, the student must have achieved the specified outcomes in the training.
Note: A PCDT certificate is issued by the SAPC once the pharmacist records their PCDT supplementary training with the SAPC.
After successful completion of the course, the PCDT pharmacist must:
| (a) | Record their supplementary training (PCDT) at the SAPC by completing and submitting the application form for SAPC Supplementary Training (online or printable form available at: http://www.pharmcouncil.co.za) together with a certified copy of the pharmacist’s ID, certified copies of the pharmacist’s PCDT certificate and proof of payment of the SAPC registration fee (refer to SAPC application form). |
| (b) | After receiving the PCDT certificate from the SAPC the PCDT pharmacist must apply for a Section 22(A)15 permit at the NDoH (the application form can be obtained by sending an e-mail to [email protected]). |
| (c) | Upon receipt of the Section 22(A)15 permit from the NDoH the PCDT pharmacist must record their PCDT permit at the SAPC (form available on the website of the SAPC (http://www.pharmcouncil.co.za). |
| (d) | Lastly the PCDT pharmacist must apply for a “Practice Number for a Primary Care Drug Therapist’’ from the Board of Healthcare Funders of Southern Africa (BHF) (the application form can be obtained by sending an e-mail to [email protected]). |
RECOGNITION OF PRIOR LEARNING
Recognition of prior learning is not applicable.
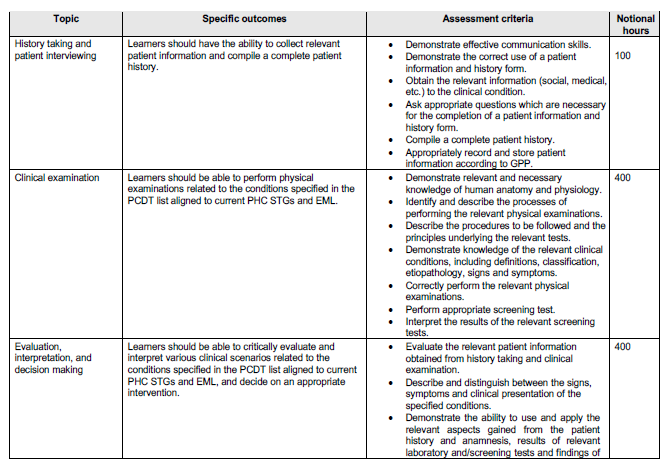
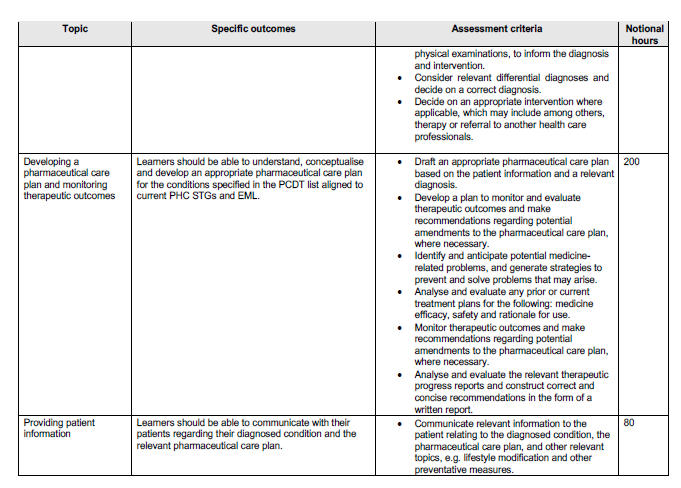
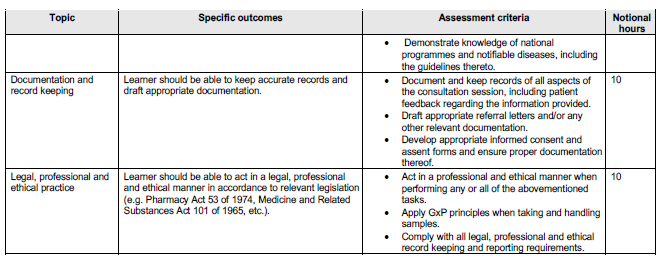
OUTCOMES AND THE ASSOCIATED ASSESSMENT CRITERIA
The minimum outcomes and assessment criteria of the course are tabulated below.
LIST OF ABBREVIATIONS BHF:
Board of Healthcare Funders of Southern Africa BPharm: Bachelor of Pharmacy GPP: Good Pharmacy Practice GxP: Is a general abbreviation for the ‘good practice’ quality guidelines, regulations, and rules, and includes Good Manufacturing Practice, Good Wholesale Practice, Good Pharmacy Practice, Good Laboratory Practice, Good Clinical Practice, etc. HEI: Higher Education Institution HPCSA: Health Professions Council of South Africa MBChB: Bachelor of Medicine and Bachelor of Surgery MCQ: Multiple Choice Questionnaire NDoH: National Department of Health OSCE: Objective Structured Clinical Examination PCDT: Primary Care Drug Therapy PHC STG and EML: Primary Health Care Standard Treatment Guidelines and Essential Medicines List PHC: Primary Health Care SAPC: South African Pharmacy Council SAQA: South African Qualifications Authority
CRITICAL CROSS-FIELD OUTCOMES
(a) Identifying and solving problems in which responses display that responsible decisions using critical and creative thinking have been made. (b) Working effectively with others as a member of a team, group, organisation, community. (c) Organising and managing oneself and one’s activities responsibly and effectively. (d) Collecting, analysing, organising and critically evaluating information. (e) Communicating effectively using visual, mathematical and/or language skills in the modes of oral and/or written persuasion. (f) Using science and technology effectively and critically, showing responsibility towards the environment and health of others. (g) Demonstrating an understanding of the world as a set of related systems by recognising that problem-solving contexts do not exist in isolation. (h) Contributing to the full personal development of each student and the social and economic development of society at large, by making it the underlying intention of any programme of learning to make an individual aware of the importance of: (i) reflecting on and exploring a variety of strategies to learn more effectively; (ii) participating as responsible citizens in the lives of local, national and global communities; (iii) being culturally and aesthetically sensitive across a range of social contexts.
QUALIFICATIONS AND EXPERIENCE OF PRESENTERS/FACILITATORS
The presenters/facilitators of the course must: (a) have an undergraduate pharmacy qualification i.e. Bachelor of Pharmacy (BPharm) degree, or recognised equivalent, plus a relevant postgraduate qualification; (b) be registered as a Pharmacist with the SAPC; (c) have completed the PCDT supplementary training; (d) have recorded their PCDT supplementary training with the SAPC; and (e) preferably be in possession of a valid Section 22A(15) permit issued by the NDoH. At least one presenter for the practical component must: (a) have a MBChB degree, or recognised equivalent; and (b) be registered as a medical practitioner at the Health Professions Council of South Africa (HPCSA); STANDARDS FOR PRESENTATION OF THE PCDT SUPPLEMENTARY TRAINING The PCDT course must be presented by a Higher Education Institution accredited with SAPC. MODE OF DELIVERY PCDT is supplementary training for pharmacists and must be presented in a manner that allow flexible study hours. Any other mode of delivery should allow flexible study hours. The HEI must have a reliable electronic platform that makes provision for sharing of study material and resources. This platform must have access control and at a minimum allows for the following: (a) General announcements; (b) Communication with students; (c) Resources and training material (including study guides, PowerPoint® presentations, video’s); (d) Submission of work assignments; and (e) Online test and examinations. A comprehensive course reader/study guide must be available for the courses. This must guide the student through the learning process and should integrate all the topics. Additional textbooks and references must also be used. At least one contact session/workshop (minimum 2 days) must form part of practical component. The purpose of the contact session is to equip the student with the necessary practical examination skills required to examine a patient. The students must attend the contact session before commencing with their 200 health examinations. ASSESSMENT OF THE PCDT SUPPLEMENTARY TRAINING The method of assessment for the courses must include both formative and summative assessment. Formative assessment in the form of work assignments and formal summative assessment by means of an examination at the end of the course should be included. The examination must be in the form of a written/online examination and the pass requirement for the theory part of the training should be 60%. The practical component can only be conducted after successful completion of this examination. The assessment of the practical component must be in the form of an OSCE. Admission to the examination is obtained through proof of participation, confirming that the leaner has met the requirement for admission to the examination.
Practical component
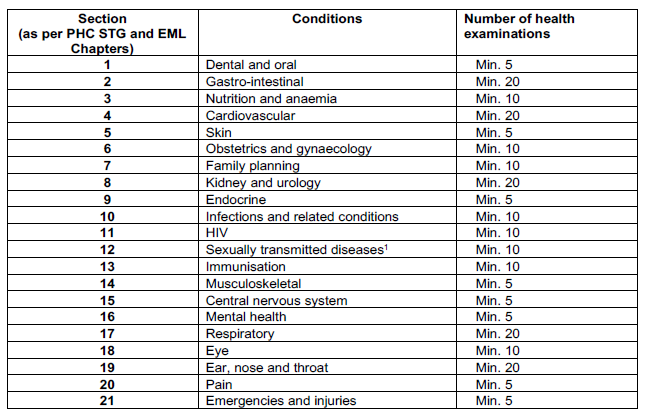
Compilation of a portfolio (containing at least 200 patient examinations on a PHC level) is an integral part of the practical component. The 200 patient examinations must include all the conditions listed in the PHC STG and EML in line with the scope of practice of a PCDT pharmacist.
The portfolio must contain at least 200 patient examinations and should include at least the following number of patient examinations per condition:
Each student’s portfolio must be evaluated prior to practical examination at the end of the course. The portfolios must meet at least the following acceptance criteria:
| (a) | contain a minimum of 200 valid patient examinations (as per minimum number listed above); |
| (b) | include intramuscular (IM) and immunisation administrations; |
| (c) | in cases where the doctor/nurse/health care professional prescribed medicine that is not within the scope of a PCDT pharmacist the student must have made a note on what he/she (as PCDT pharmacist) would have done if this patient came to the pharmacy clinic (including referral to a doctor). |
| (d) | Each health examination must include the correct medication (that a PCDT pharmacist may prescribe as per the PCDT list), medicine strength, dosage and duration of treatment. (e) Health examination which are not on a PHC level do not count as suitable cases for the portfolio. The student must be able to distinguish between: |
| (i) | medication which is regularly available in the pharmacy as OTC medication; |
| (ii) | medication allowed to be prescribed by the PCDT pharmacist (in accordance to the PCDT list and PHC STG and EML); and |
| (iii) | medication which may only be prescribed by a medical practitioner (doctor’s prescription). Portfolios that do not meet the requirements may be returned to the student for correction/ additional health examinations and may be submitted by the student before the next practical examination opportunity. |
Portfolios that do not meet the requirements may be returned to the student for correction/ additional health examinations and may be submitted by the student before the next practical examination opportunity.
PROCESS OF APPEAL
A process must be in place in cases where students disagree with the outcome of an assessment (written of practical). Appeals against assessment decisions on the demonstration of competence by candidates must be described in the study guide of the course.
PROCESS IN CASE OF DISHONESTY AND PLAGIARISM
Students must be warned against dishonesty and plagiarism. A procedure must be in place to address this kind of misconduct and in serious cases, should be reported to the SAPC.
CERTIFICATION METHODS AND PROCEDURES
Procedures must be in place to ensure that certification of the students is managed in a secure and safe manner. The security and accuracy of certificates during printing, filing and distribution must be assured. The following minimum information is required for certification of the course:
| (a) | provider name and/or logo |
| (b) | name of the course |
| (c) | student’s full name (first names followed by surname) |
| (d) | date of issue of the certificate |
| (e) | signatories |
FACILITIES, EQUIPMENT AND CONSUMABLES
The physical facilities of the HEI must be adequate. Essential physical facilities must include offices for administrative staff and course presenters, lecture rooms and clinical simulation laboratory (for presentation of compulsory contact session/workshops). The facilities must be adequately equipped to at least cover the conditions specified in the PCDT list aligned to current PHC STGs and EML, well maintained and provide a reasonably attractive environment for teaching and learning.
The facilities, including lecture rooms and clinical simulation laboratory, must be sufficient in number and adequate in size to accommodate the number of students. The clinical simulation laboratory must also provide adequate storeroom facilities for housing of equipment and supplies and must include areas for practice simulations and an area(s) where the practical examination can be held.
The equipment in the clinical simulation laboratory must include (in sufficient amounts) at least the following:
| (a) | hospital bed/patient examination bed, |
| (b) | stethoscope, |
| (c) | otoscope (with different size earpieces), |
| (d) | sphygmomanometer or calibrated blood pressure apparatus, |
| (e) | tuning fork, |
| (f) | blood glucose meter, lancets and test strips, |
| (g) | cholesterol meter, |
| (h) | peak flow meter and disposable mouth pieces, |
| (i) | tongue depressors, |
| (j) | disposable rubber gloves, |
| (k) | clinical thermometer, |
| (l) | alcohol swabs of 70% alcohol solution or surgical spirits, |
| (m) | towels or paper towels, |
| (n) | disinfectants for equipment, |
| (o) | soap and water for washing of hands, |
| (p) | applicable equipment for approved screening tests, |
| (q) | mirror, |
| (r) | scale plus baby scale, |
| (s) | height chart and tape measure, and |
| (t) | equipment for first aid, e.g. plaster, gauze, cotton wool, sterile wound dressings, scissors. |
STANDARDS FOR ADMINISTRATION AND RECORD KEEPING
A student administration system must be available for maintaining and updating detailed information about each enrolled student. Information must include but not be limited to the following:
| (a) | student’s full names and surname, |
| (b) | maiden name (if applicable), |
| (c) | identification number, |
| (d) | cell phone number, |
| (e) | e-mail address, |
| (f) | postal address, |
| (g) | qualifications, and |
| (h) | past employment (indicating work experience in a clinical environment). |
The system must include a functionality to generate a document that can be used as “Proof of Registration” for each enrolled student.
The student administration system must also allow for record keeping of the marks that each student has obtained for the course and must include a functionality to generate an “Academic Record” for each student. Confidentiality of personal information must be maintained at all times.
| 6. | APPENDIX A - PCDT List |
Latest List must be used at all times and must be obtained from the Department of Health.
__________________________________________________________
1 Syndromic STI diagnosis and treatment, no genitourinary examination is allowed, as it does not form part of the scope of practice of a PCDT pharmacist.