 Auditing Profession Act, 2005
Auditing Profession Act, 2005
R 385
Occupational Health and Safety Act, 1993 (Act No. 85 of 1993)RegulationsRegulations for Hazardous Biological Agents, 2022AnnexuresAnnexure A: Categorisation of Biological Agents according to Risk Group |
ANNEXURE A
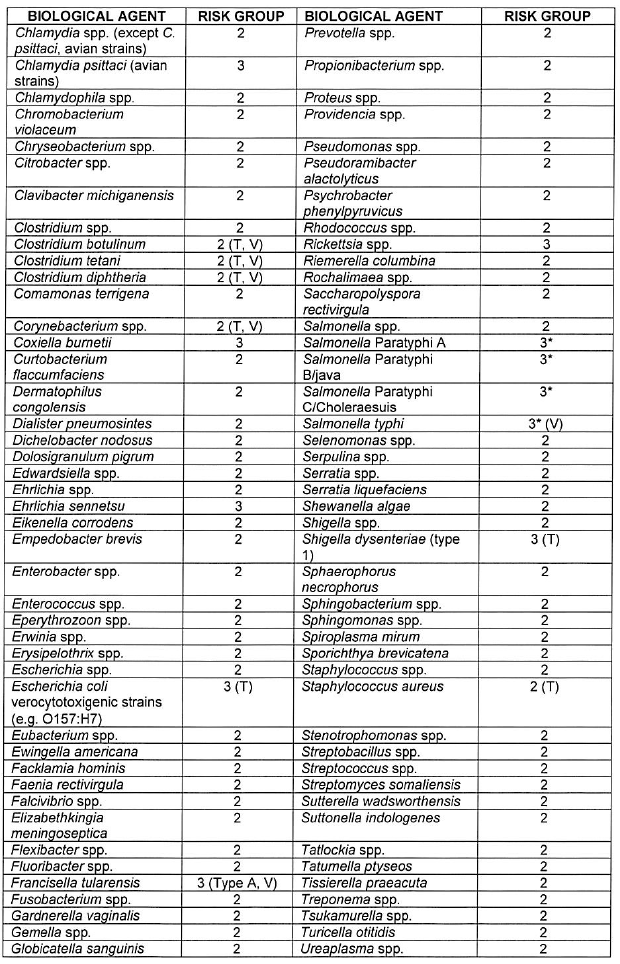
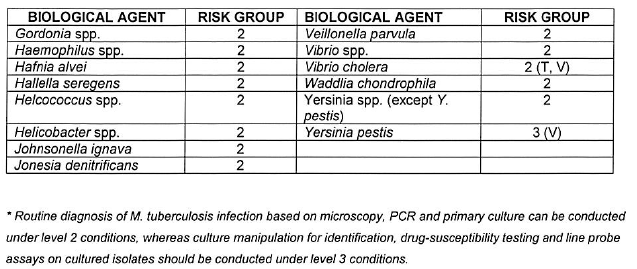
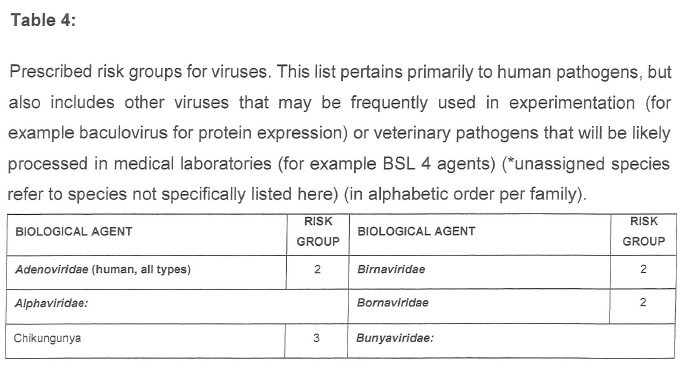
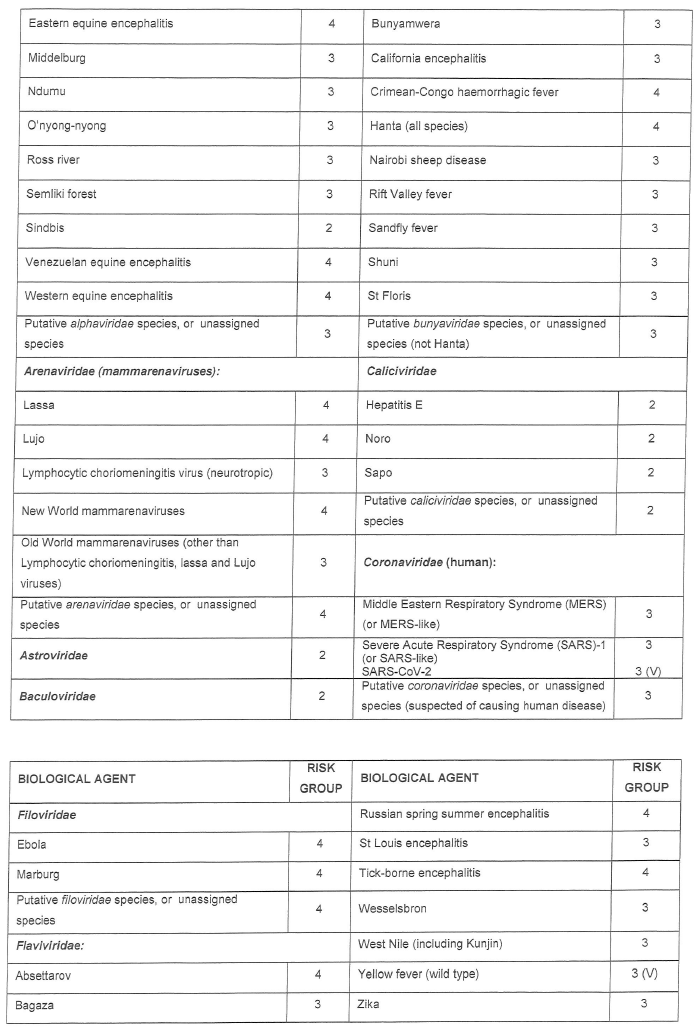
CATEGORISATION OF BIOLOGICAL AGENTS ACCORDING TO RISK GROUP
INTRODUCTION
| 1. | Biological agents listed are categorised into the following risk groups on the basis of their ability to cause human disease by infection, allergy and/or toxicity, potential to cause epidemics or pandemics, endemicity in South Africa and availability of curative or prophylactic treatment: |
| (a) | Risk Group 1 HBA, an HBA that is unlikely to cause human disease; |
| (b) | Risk Group 2 HBA, an HBA that may cause human disease and be a hazard to exposed persons, which is unlikely to spread to the community and for which effective prophylaxis and treatment is usually available; |
| (c) | Risk Group 3 HBA, an HBA that may cause severe human disease, which presents a serious hazard to exposed persons and which may present a risk of spreading to the community, but for which effective prophylaxis and treatment may be available; and |
| (d) | Risk Group 4 HBA, an HBA that cause severe human disease and is a serious hazard to exposed persons and which may present a high risk of spreading to the community, but for which no effective prophylaxis and treatment is available. |
[Annexure A (1) substituted by Notice No. R. 2693 of GG47413, dated 31 October 2022]
| 2. | In allocating biological agents to a risk group, account is not taken of effects on those whose susceptibility may be affected for one or other reason such as pre-existing disease, medication, compromised immunity, pregnancy or breastfeeding. Workplace specific risk to such workers should be considered per risk assessment as in regulation 6. |
| 3. | Biological agents that have not been classified for inclusion in groups 2 to 4 of the list are not implicitly classified as Group 1. All viruses that have been isolated in humans and that have not been assessed and allocated to a group in the list are to be classified in group 2 as a minimum, except where there is evidence that they are unlikely to cause disease in humans. |
| 4. | If more than one species of any particular agent is known to be pathogenic to humans, the most prominent of these is generally named, together with the wider reference "species" (spp.) to indicate the fact that the other species of the same genus may be hazardous. If a whole genus is mentioned in this way, it is implicit that species and strains that are non-pathogenic to humans are excluded. |
| 5. | When a strain is attenuated or has lost known virulence genes, then the containment required by the classification of its parent strain need not necessarily apply, subject to risk assessment as per regulation 6, for example, when such strain is used as a product or as part of a product for prophylactic or therapeutic purposes (see point 2). |
| 6. | The requirements as to containment consequent upon the classification of parasites apply only to stages in the life cycle of the parasite in which it is liable to be infectious, allergic or toxic to humans. |
| 7. | The list also gives a separate indication where biological agents are capable of causing allergic or toxic reactions, and where a registered vaccine is available for use in the Republic of South Africa. |
The indications are identified by the following notations:
| • | A: possible allergic effects; |
| • | T: toxin production; and |
| • | V: vaccine available. |
| 8. | The selection of control measures for biological agents should take into account the fact that there are no exposure limits for them. Their ability to replicate and to infect,cause allergic or toxic effects, at very low doses, means that exposure may have to be reduced to levels that are diminishingly low. |
For each activity the first consideration should be whether it can be carried out in a way that involves exposure to a less harmful biological agent. This may be practicable, for example, in teaching and some types of research. If there is more than one way of carrying out the activity, then the method carrying the least risk should be chosen.
If the least harmful alternative still involves exposure or potential exposure to a biological agent, or the nature of the activity is such that there is no choice, and it is not reasonably practicable to prevent exposure by some other means, then exposure should be adequately controlled.
| 9. | Agents with reduced virulence may be used at a lower than normal level of containment if the alteration has effectively changed their classification. |
A biological agent that falls or is treated as falling into hazard Group 1 may be a Group 3 genetically modified organism because of environmental risks associated with it or because, though now unlikely to cause human disease, it is derived by genetic modification from a pathogenic parental organism. In the latter case, the selection of containment measures appropriate to the agent's reduced virulence and corresponding group may be permitted. Where there is a mismatch, as in the case of a genetically modified organism or biological agent that is non-hazardous to humans but environmentally harmful, the more stringent requirements should be followed.
Where the rules set out lead to a particular containment level for an activity, all the measures appropriate to that level should normally be used. Some selection may be done, however, to suit individual circumstances, provided that by doing so the risk is not increased.
Regulation 11 sets out additional requirements in respect of personal protective equipment used to protect employees against biological agents. The objective of these requirements is to prevent the equipment itself from acting as the means by which agents are transmitted, and they should be followed accordingly.
Where workers are exposed to biological agents, the information and instruction given to them, if applicable, should be set down in the form of written instructions, outlining procedures to be followed after a serious incident involving the handling of a biological agents as well as the procedure for handling any Group 4 agent.
If the nature of the workplace and the activity are such that employees may need instant access to this information, or where a reduction in risk may be expected by having the information conspicuously displayed in the workplace then it should also be set out on notices displayed in the workplace.
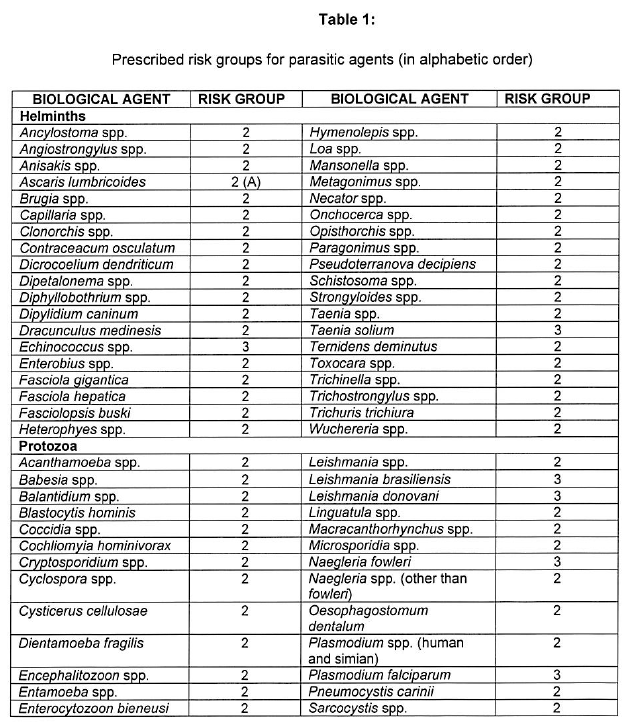

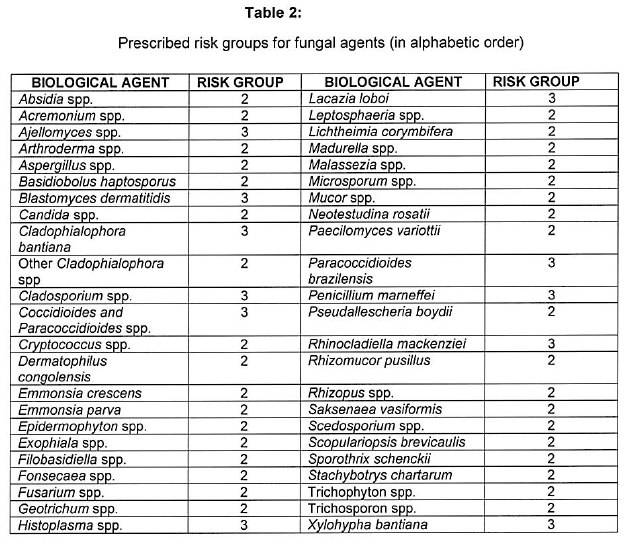
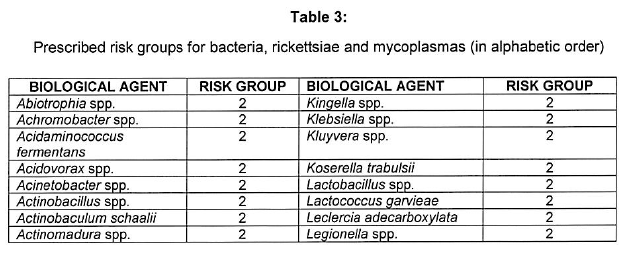
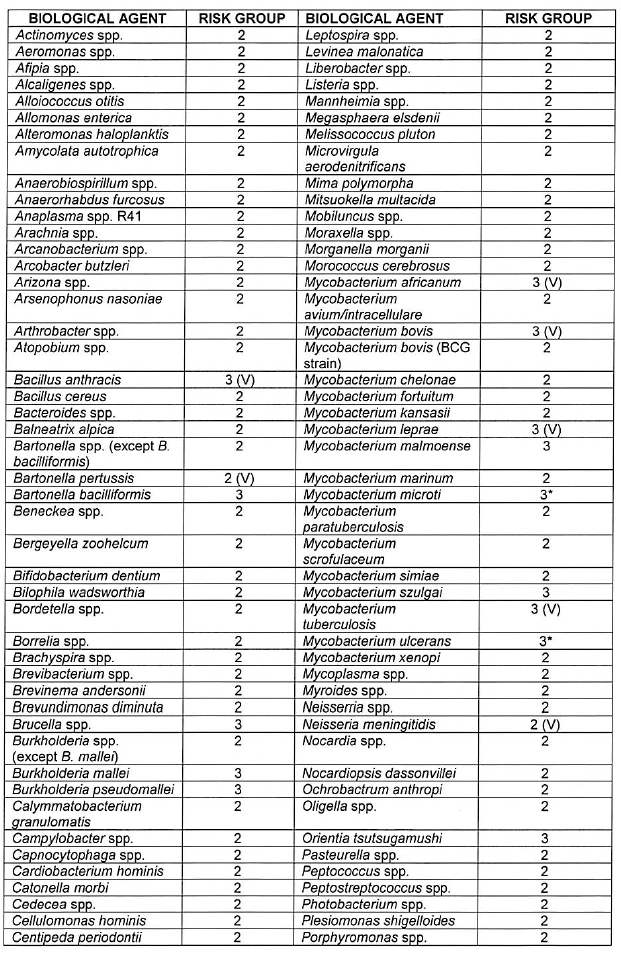
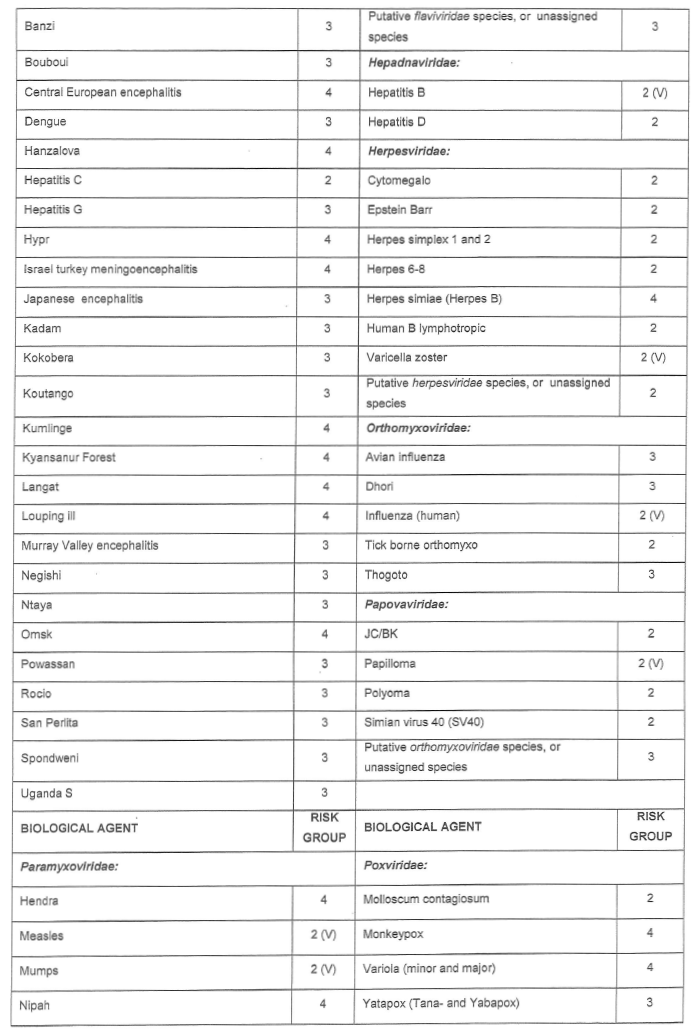
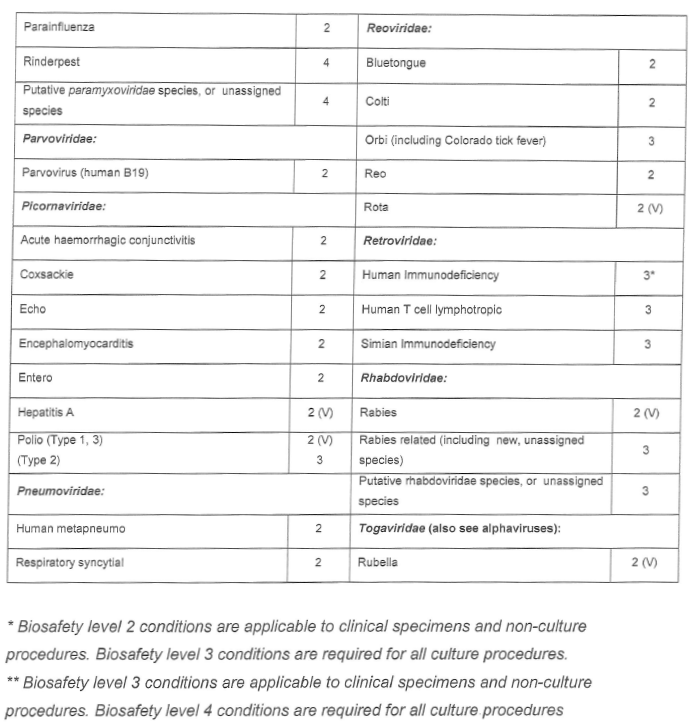
[Annexure A Table 4 inserted by Notice No. R. 2693 of GG47413, dated 31 October 2022]